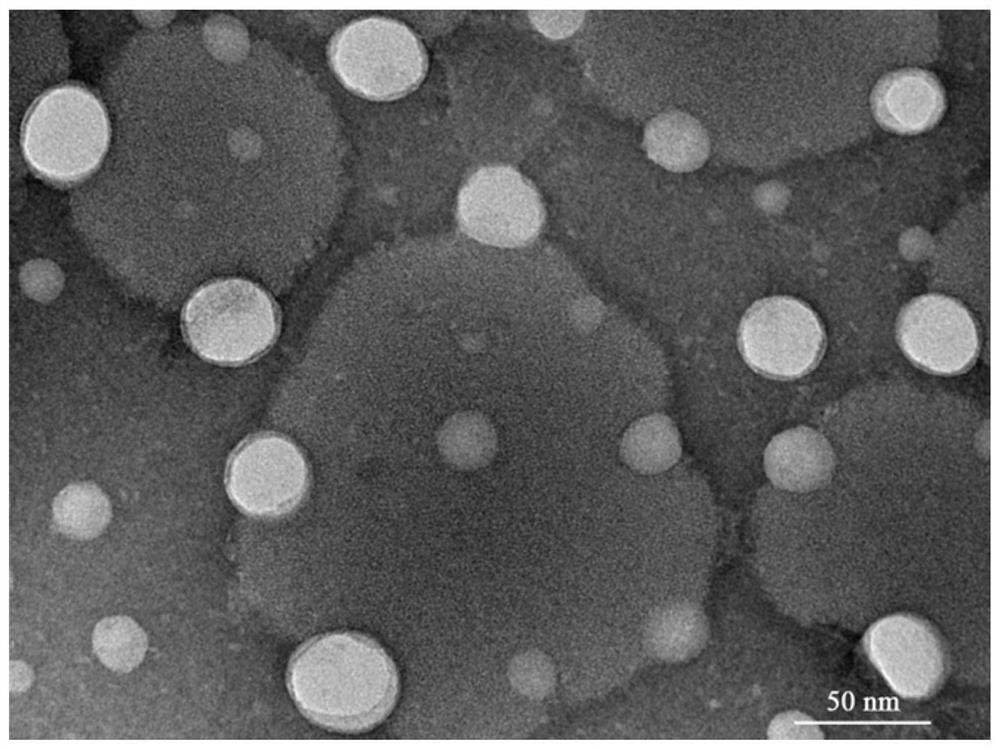
A kind of chitosan lipoprotein nasal administration nanocomposite and its preparation method and application
A nano-composite and nasal drug delivery technology, which is applied in nano-drugs, drug combinations, drug delivery, etc., can solve problems such as burden, and achieve the effects of good absorption, high-efficiency brain targeting, and smooth and round appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Weigh 0.5 g of chitosan (molecular weight is 150KDa, deacetylation degree is 98%) and dissolve it in 60 mL of 0.5% acetic acid solution. Another 4-carboxyphenylboronic acid (PBA) and 206.6 mg of N-hydroxysuccinimide (NHS) with 0.45 times the molar amount of chitosan units were weighed and dissolved in 30 mL of methanol, and stirred at room temperature for 30 min. 342.57 mg of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC·HCl) was weighed and added to the PBA / NHS mixed solution, and the mixture was stirred to dissolve uniformly. Then the PBA / NHS / EDC·HCl mixed solution was added to the acetic acid solution of chitosan (the volume ratio of methanol and water in the reaction system was 1:2), stirred at room temperature for 24 hours, and the reaction solution was transferred to a molecular weight cut-off of 14 ×10 3 Dialyzed in deionized water for 72h, changing the water every 4h. The dialyzed samples were freeze-dried to obtain a light yellow powdery chitosan-phenyl...
Embodiment 2
[0046] With reference to the process conditions of Example 1, chitosan (molecular weight is 150KDa, deacetylation degree is 98%) is adjusted to chitosan (molecular weight is 150KDa, deacetylation degree is 60%) to obtain chitosan-phenylboronic acid For the grafted polymer, the graft ratio of 9% was obtained by calculating the peak area of NMR.
Embodiment 3
[0048] With reference to the process conditions of Example 1, chitosan (molecular weight is 150KDa, deacetylation degree is 98%) is adjusted to chitosan (molecular weight is 150KDa, deacetylation degree is 80%) to prepare chitosan-phenylboronic acid For the grafted polymer, the NMR peak area calculation method was used to obtain a graft ratio of 12%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com