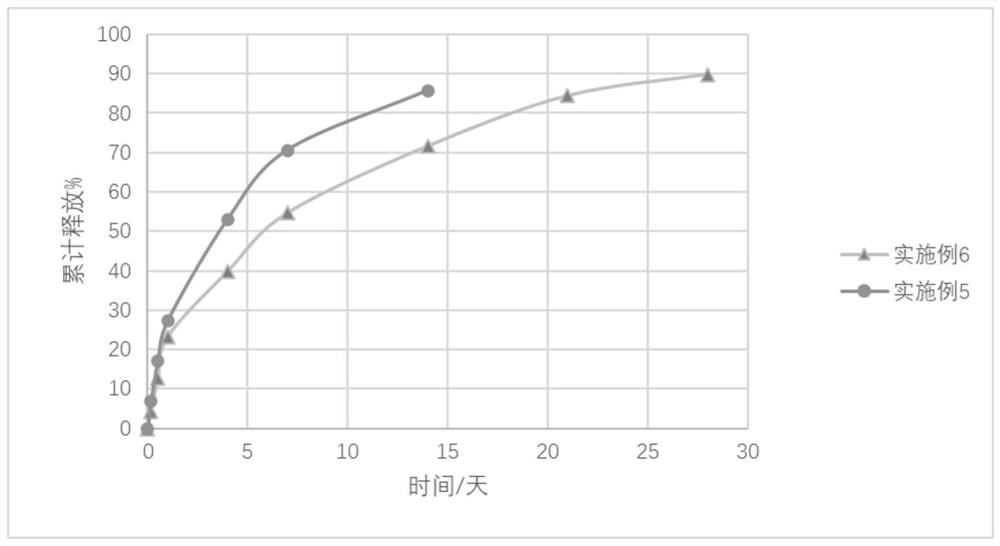
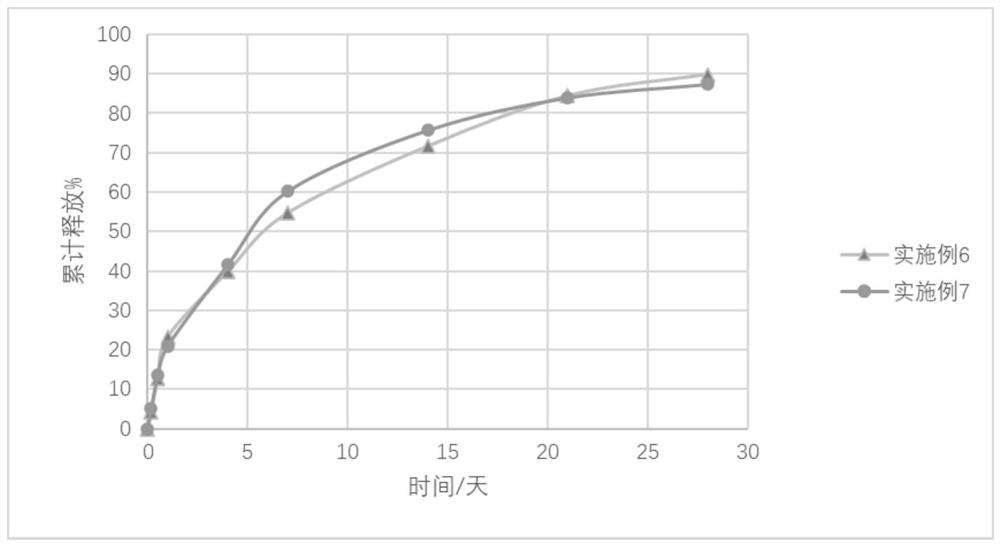
Long-acting injection gel containing entecavir
An entecavir and gel technology, applied in the field of long-acting injection gel, can solve the problems of only 16% bioavailability, low oral drug penetration rate, and inability to stop the drug casually, so as to reduce the probability of virus rebound and solve gastrointestinal problems. The effect of reducing side effects and improving medication compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Take two parts of 150mg PLGA solids with uniform size, the specification is monomer ratio LA:GA=50:50, Mw is 40KDa, add 350mg of N-methylpyrrolidone or 350mg of ethyl acetate respectively, place in a beaker, Stir and dissolve at room temperature at 500 rpm for 60 min. Observe the dissolution phenomenon, and drop the formed liquid into the water to observe the phenomenon.
[0028] It can be seen that NMP can dissolve the added PLGA in about 30 minutes to form a clear solution with a certain viscosity, but ethyl acetate forms a suspension in 60 minutes. Drop the two liquids formed into water respectively. It can be seen that both of them will gel into small balls, but there are flocs in the PLGA dissolved in ethyl acetate. It shows that NMP is more suitable for forming gel with PLGA.
Embodiment 2
[0030] Take three parts of 150mg PLGA solid with uniform size, the specification is monomer ratio LA:GA=50:50, Mw is 40KDa, add 600mg, 400mg and 225mg of N-methylpyrrolidone respectively, put in a beaker, Stir and dissolve at 500 rpm for 60 minutes to make 20%, 27%, and 40% PLGA solutions, observe the dissolution phenomenon, and drop the formed liquid into water to observe the phenomenon.
[0031] It can be seen that NMP can dissolve the added 27% PLGA within about 30 minutes, 20% needs 15 minutes to dissolve, and 40% needs about 60 minutes to dissolve, forming a clear gel solution with a certain viscosity. The viscosity of the gel solution was the largest for the 40% PLGA solution, followed by the 27% one. The contact time with hydrogel is 6 seconds, 3 seconds and 1 second respectively. The effect of coagulation is that 20% has a certain viscosity and softness, and 40% has the strongest character and the least softness.
Embodiment 3
[0033] Take three parts of 150mg PLGA solid with uniform size, the specification is monomer ratio LA:GA=50:50, Mw is 20KDa, 30KDa, 50KDa respectively, add 350mg of N-methylpyrrolidone respectively, put it in a beaker, at room temperature Stir and dissolve at 500rpm for 60min to make a PLGA solution, observe the dissolution phenomenon, and drop the formed liquid into water to observe the phenomenon.
[0034] It can be seen that the dissolution time is 20min, 25min, and 50min respectively; in the case of gel, PLGA with 50KDa has the highest viscosity, and PLGA with 20KDa has the lowest viscosity; it coagulates quickly when it meets water. As the molecular weight of PLGA increases, the coagulation time becomes shorter.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com