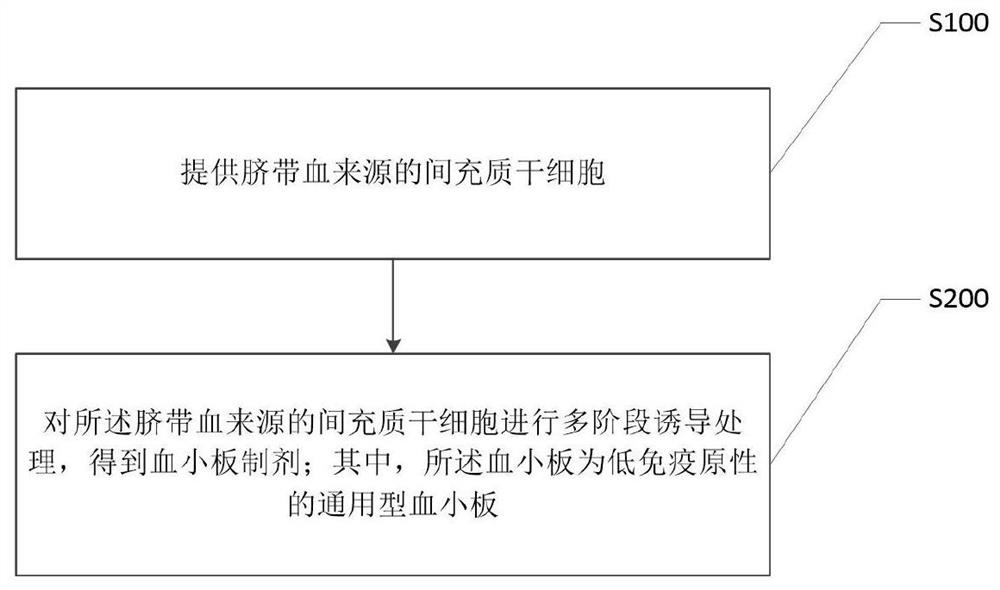
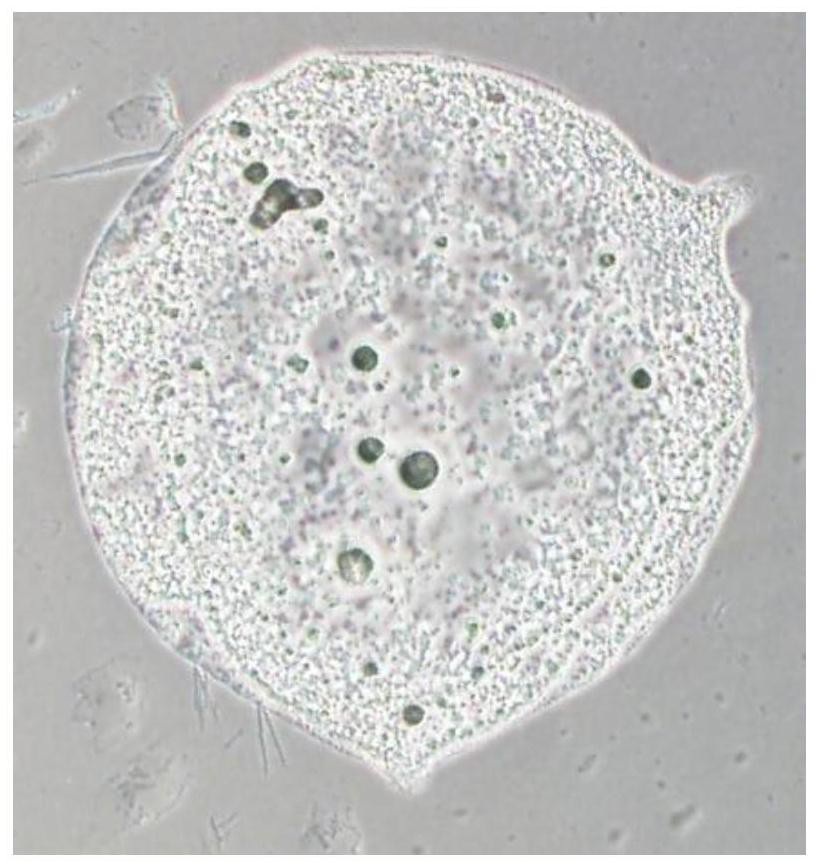
Universal platelet preparation prepared from umbilical cord-derived mesenchymal stem cells and method
A technology for stem cells and platelets, which can be used in cell culture active agents, biochemical equipment and methods, animal cells, etc., and can solve the problems of inconvenient preparation and storage, poor universality of platelets, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1. Preparation of Umbilical Cord Mesenchymal Stem Cells
[0048] 1.1 With informed consent, fresh umbilical cords were obtained for stripping to obtain Wharton's jelly, and after the Wharton's jelly was cut into pieces, primary cell culture was carried out in a serum-free culture system by using the adherent method. The serum-free culture system For: DMEM / F11 + commercially available MSCs culture factors.
[0049] 1.2 The primary umbilical cord mesenchymal stem cells are amplified, the cell generation is between P3-P5, and they are used to induce transformation to adipogenicity. The day before induction (Day-1), the cell fusion degree should be greater than 85% and Cultured at 37°C, 5% CO 2 in the incubator.
[0050] 2. Induction phase
[0051] 2.1 Adipogenic induction stage
[0052] 2.1.1 When adipogenic induction is started (Day0), the serum-free culture system is completely replaced with the adipogenic induction medium, and the cells are cultured for 10 days. The...
Embodiment 2
[0063] 1. Preparation of Umbilical Cord Mesenchymal Stem Cells
[0064] 1.1 With informed consent, fresh umbilical cords were obtained for stripping to obtain Wharton's jelly, and after the Wharton's jelly was cut into pieces, primary cell culture was carried out in a serum-free culture system by using the adherent method. The serum-free culture system For: DMEM / F11 + commercially available MSCs culture factors.
[0065] 1.2 The primary umbilical cord mesenchymal stem cells are amplified, the cell generation is between P3-P5, and they are used to induce transformation to adipogenicity. The day before induction (Day-1), the cell fusion degree should be greater than 85% and Cultured at 37°C, 5% CO 2 in the incubator.
[0066] 2. Induction phase
[0067] 2.1 Adipogenic induction stage
[0068] 2.1.1 When adipogenic induction is started (Day0), the serum-free culture system is completely replaced with the adipogenic induction medium, and the cells are cultured for 16 days. The...
Embodiment 3
[0079] 1. Preparation of Umbilical Cord Mesenchymal Stem Cells
[0080] 1.1 With informed consent, fresh umbilical cords were obtained for stripping to obtain Wharton's jelly, and after the Wharton's jelly was cut into pieces, primary cell culture was carried out in a serum-free culture system by using the adherent method. The serum-free culture system For: DMEM / F11 + commercially available MSCs culture factors.
[0081] 1.2 The primary umbilical cord mesenchymal stem cells are amplified, the cell generation is between P3-P5, and they are used to induce transformation to adipogenicity. The day before induction (Day-1), the cell fusion degree should be greater than 85% and Cultured at 37°C, 5% CO 2 in the incubator.
[0082] 2 induction phase
[0083] 2.1 Adipogenic induction stage
[0084] 2.1.1 When adipogenic induction is started (Day0), the serum-free culture system is completely replaced with the adipogenic induction medium, and the cells are cultured for 13 days. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com