Fluoro-boron formazan near-infrared-II fluorescent dye, preparation method and application
A fluorescent dye and fluorescent probe technology, applied in the field of preparing the fluorescent dye, can solve the problems of limited synthesis steps, high synthesis cost, low reaction yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
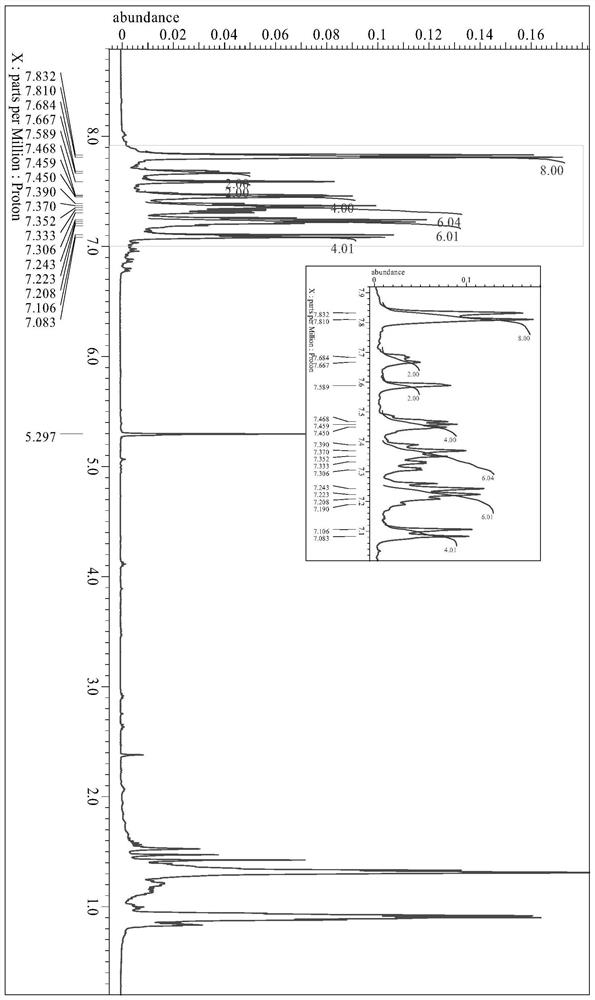
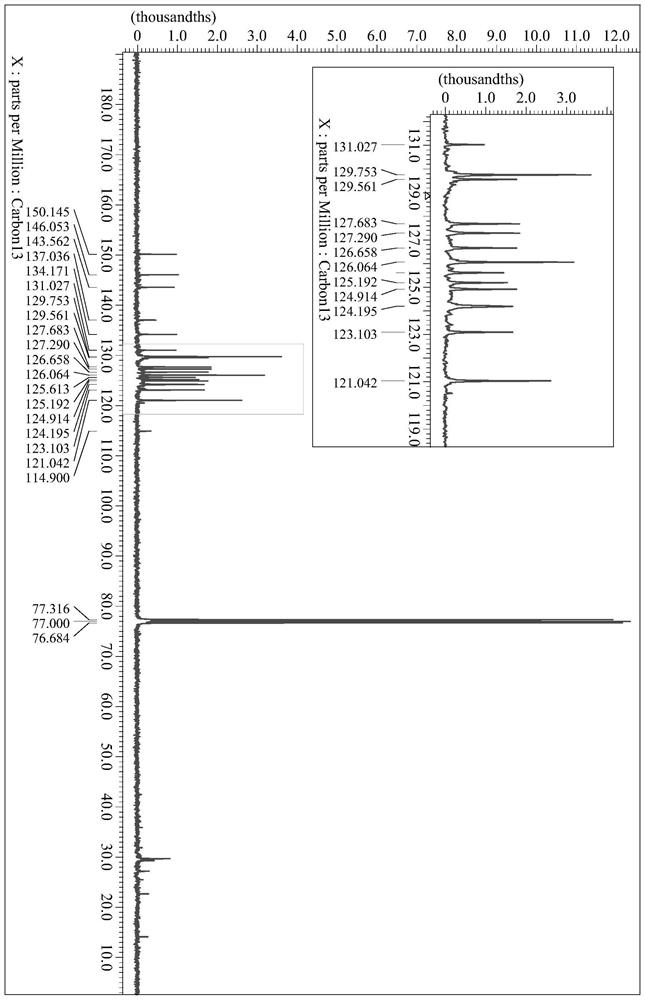
[0036] Synthesis of embodiment 1 formula (I) compound
[0037] The synthetic route is as follows:
[0038]
[0039] The specific operation steps are as follows:
[0040] (1) Choose a two-necked flask (500mL), dissolve 1-naphthylaminobenzene (10.96g, 50mmol) and potassium carbonate (13.79g, 100mmol) in dimethyl sulfoxide (200mL), add p-fluoronitrobenzene ( 5.30mL, 50mmol), protected under nitrogen atmosphere, heated and refluxed at 150°C for 48 hours, cooled to room temperature, extracted with ethyl acetate, washed with saturated brine to remove excess DMSO, and distilled under reduced pressure to obtain a brown oily crude product, which was chromatographed on silica gel Column purification (dichloromethane:petroleum ether=1:4) gave an orange oily semi-solid, i.e. intermediate (II) (5.78g, yield 34%), and its chemical structural formula is as follows:
[0041]
[0042](2) Select a 250mL two-necked flask, dissolve the intermediate (II) (4.68g, 13.76mmol) and Pd / C catalys...
Embodiment 2
[0047] Embodiment 2 formula (I) compound BDF-Na nanoparticle preparation
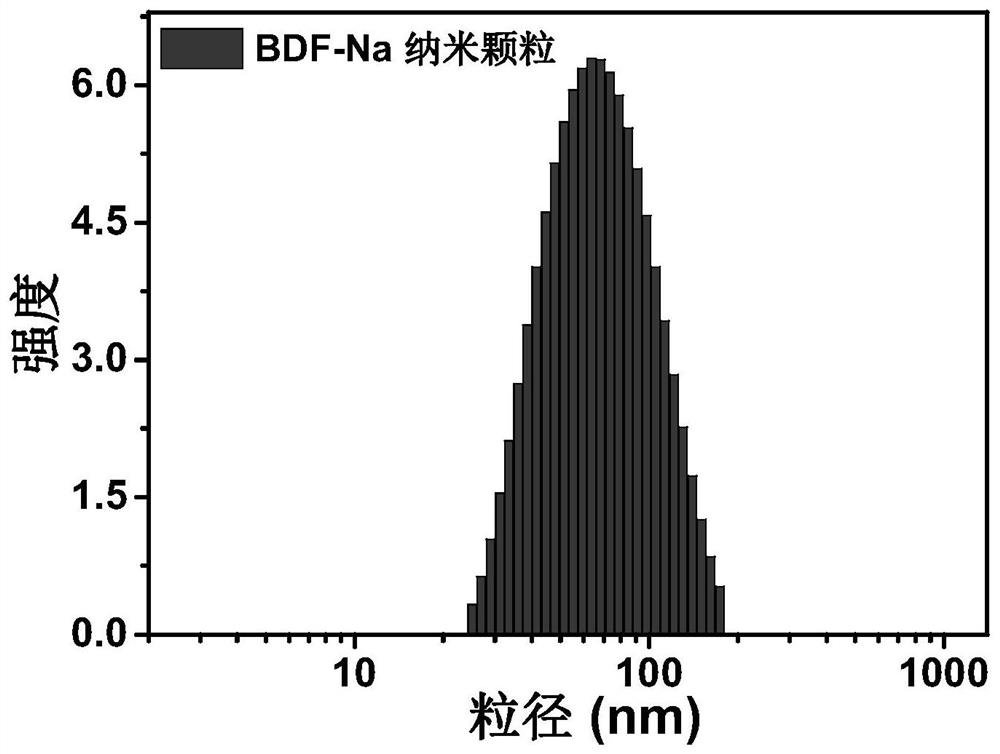
[0048] Dissolve the BDF-Na dye of the compound of formula (I) synthesized above in 1 mL of tetrahydrofuran, quickly inject it into the PBS (10 mL) solution containing the amphiphilic matrix poly(styrene)-block-poly(ethylene glycol), and sonicate for 10 minutes, stirred at room temperature for 12 hours, and removed the organic solvent to obtain nano-functionalized BDF-Na organic nanoparticles. as attached image 3 The dynamic light scattering test results of the organic nanomaterial BDF-Na nanoparticles shown show that the hydrated particle size is about 70nm, and the nanoparticles of this size can effectively penetrate the cell membrane and enter the cell smoothly.
Embodiment 3
[0049] Embodiment 3 adopts formula (I) compound BDF-Na photophysical performance test prepared by the present invention
[0050] Add formula (I) compound BDF-Na dichloromethane solution (2.5mL) in the quartz cuvette of ultraviolet test, concentration is 1 * 10 -5 M, test its UV absorption spectrum. The results are attached Figure 4 Shown, the maximum ultraviolet absorption wavelength of formula (I) compound BDF-Na is 770nm in the near-infrared absorption range;
[0051] Add formula (I) compound BDF-Na dichloromethane solution (2.5mL) in the quartz cuvette of fluorescence test, concentration is 1 * 10 -5 M, to test its fluorescence emission spectrum. as attached Figure 5 As shown, the maximum fluorescence emission wavelength of the compound BDF-Na of formula (I) is 1017nm, and it absorbs in the second near-infrared region. In addition, using IR1061 (1.7%, dichloromethane solution; Chem. Phys. Lett. 2003, 373, 372-378) as a reference, the measured fluorescence quantum yie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydrated particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com