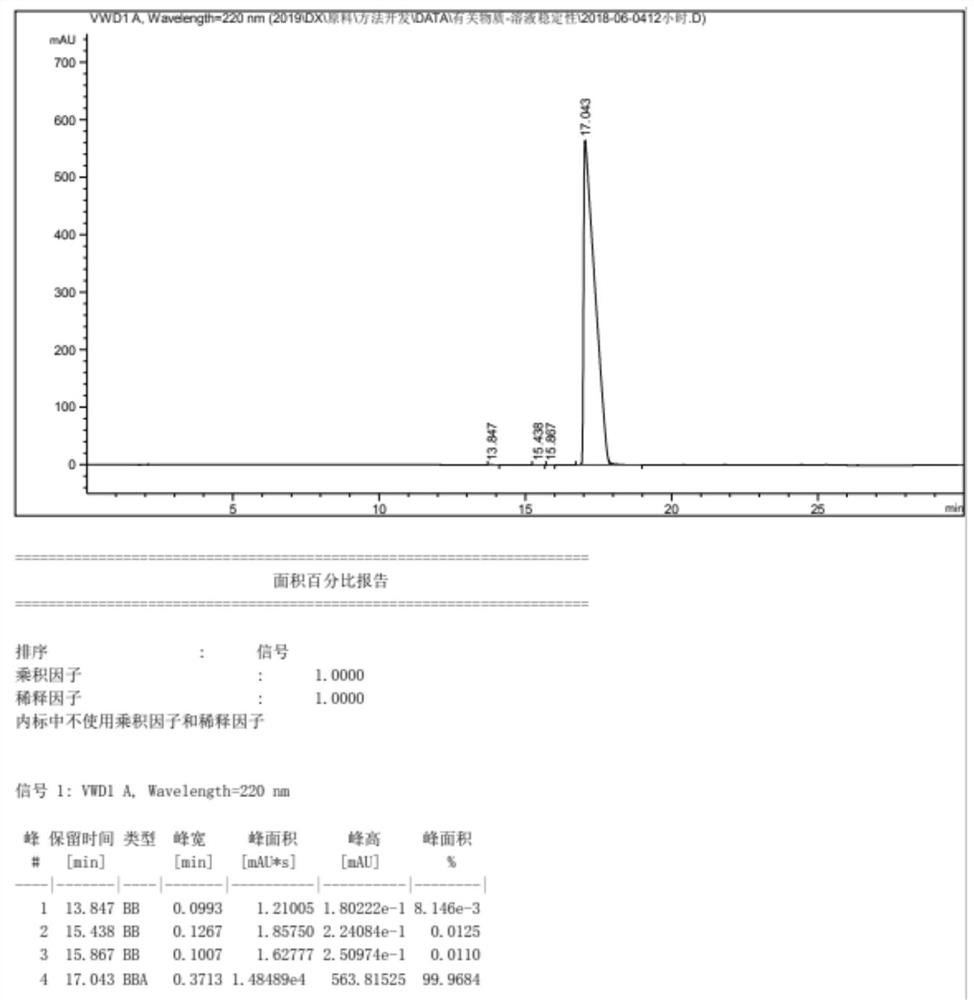
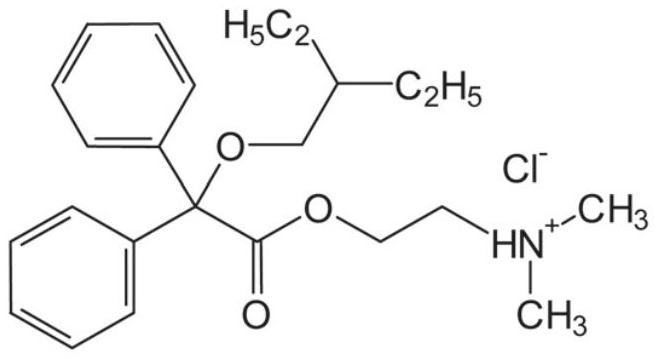
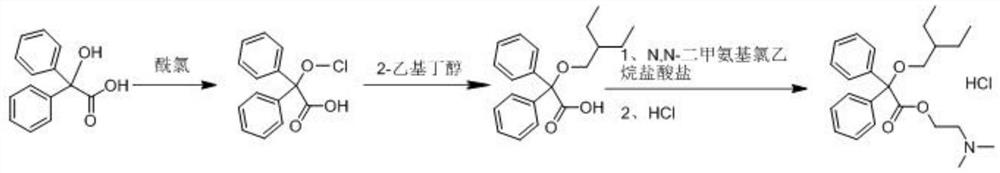
Synthesis method of dinaverine hydrochloride
A technology of dinaverine and its synthetic method, applied in chemical instruments and methods, preparation of carboxylate, preparation of organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Dinavirine hydrochloride of the present embodiment is obtained by following synthetic method:
[0028] S1. Add 2.86kg of acetonitrile and 4.10kg of 2,2-diphenyl-2-hydroxyacetic acid into a dry and clean reaction kettle, stir to dissolve, add 1.57kg of acetyl chloride, after the addition, stir and react at 25°C for 20 hours . After the reaction was completed, concentrate under reduced pressure until no solvent was evaporated, suck in 13.60 kg of toluene, add 1.80 kg of potassium carbonate, stir for 40 min, add 2.00 kg of anhydrous sodium sulfate and stir for dehydration, collect the filtrate by filtration, and wash the filter cake with 3.00 kg of toluene. and collect the wash solution. Combine the filtrate and the washing liquid, add 10.42 kg of potassium carbonate, and add 1.90 kg of 2-ethylbutanol. After the addition is completed, the temperature is raised to 100° C., and the reaction is stirred for 20 hours. After the reaction is complete, cool down to room temperat...
Embodiment 2
[0032] Dinavirine hydrochloride of the present embodiment is obtained by following synthetic method:
[0033] S1. Add 2.92kg of dichloromethane and 4.10kg of 2,2-diphenyl-2-hydroxyacetic acid into a dry and clean reaction kettle, stir to dissolve, add 1.89kg of propionyl chloride, and stir at 30°C to react 19.5 hours. After the reaction was completed, concentrate under reduced pressure until no solvent was evaporated, suck in 13.70 kg of toluene, add 1.52 kg of sodium carbonate, stir for 45 min, add 1.8 kg of anhydrous magnesium sulfate and stir for dehydration, collect the filtrate by filtration, and wash the filter cake with 2.85 kg of toluene. and collect the wash solution. Combine the filtrate and washing liquid, add 10.25 kg of sodium carbonate, and add 1.95 kg of 2-ethylbutanol. After the addition is completed, the temperature is raised to 105° C., and the reaction is stirred for 19.5 hours. After the reaction is complete, cool down to room temperature, filter, add 19....
Embodiment 3
[0036] Dinavirine hydrochloride of the present embodiment is obtained by following synthetic method:
[0037] S1. Add 2.45kg of chloroform and 4.10kg of 2,2-diphenyl-2-hydroxyacetic acid into a dry and clean reaction kettle, stir to dissolve, add 1.98kg of butyryl chloride, after the addition, stir and react at 20°C for 21 hours . After the reaction is complete, concentrate under reduced pressure until no solvent is evaporated, draw in 13.50 kg of toluene, add 1.25 kg of magnesium carbonate, stir for 35 minutes, add 1.65 kg of anhydrous calcium chloride and stir for dehydration, collect the filtrate by filtration, and wash the filter cake with 3.20 kg of toluene , and collect the wash solution. Combine the filtrate and the washing liquid, add 10.28 kg of magnesium carbonate, and add 1.92 kg of 2-ethylbutanol. After the addition is completed, the temperature is raised to 95° C., and the reaction is stirred for 21 hours. After the reaction is complete, cool down to room temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com