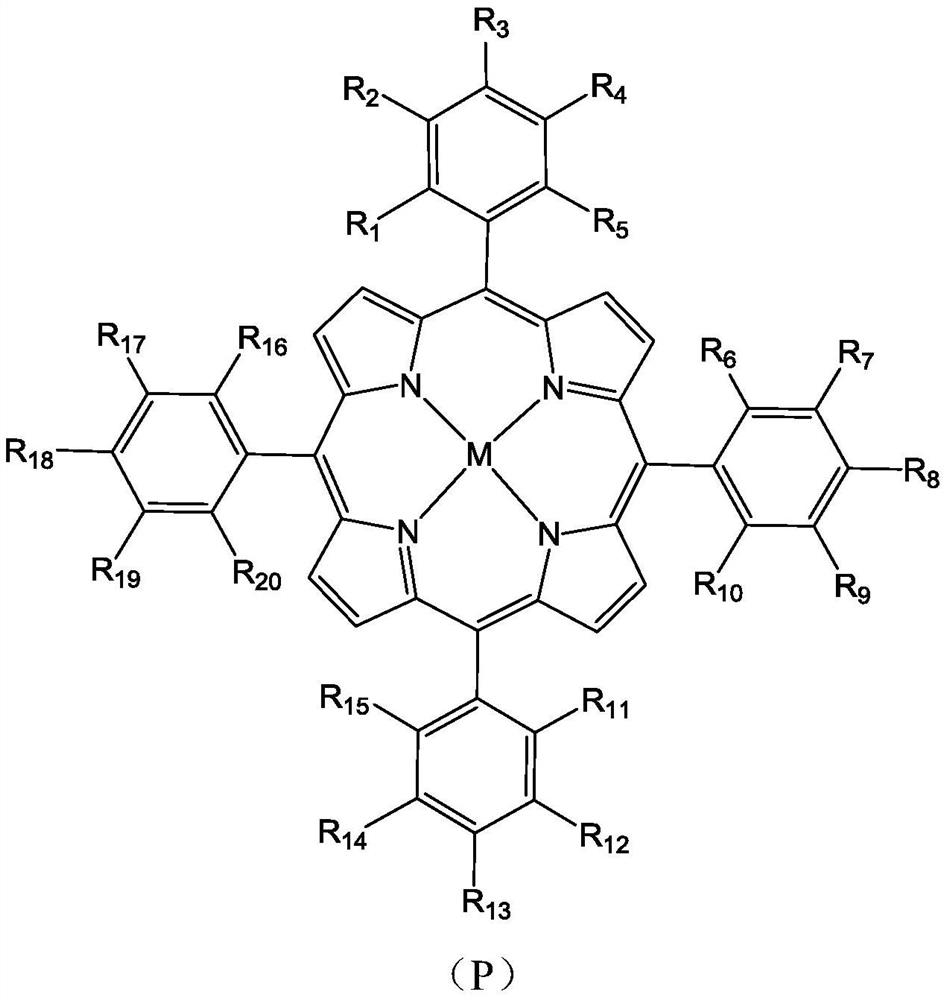
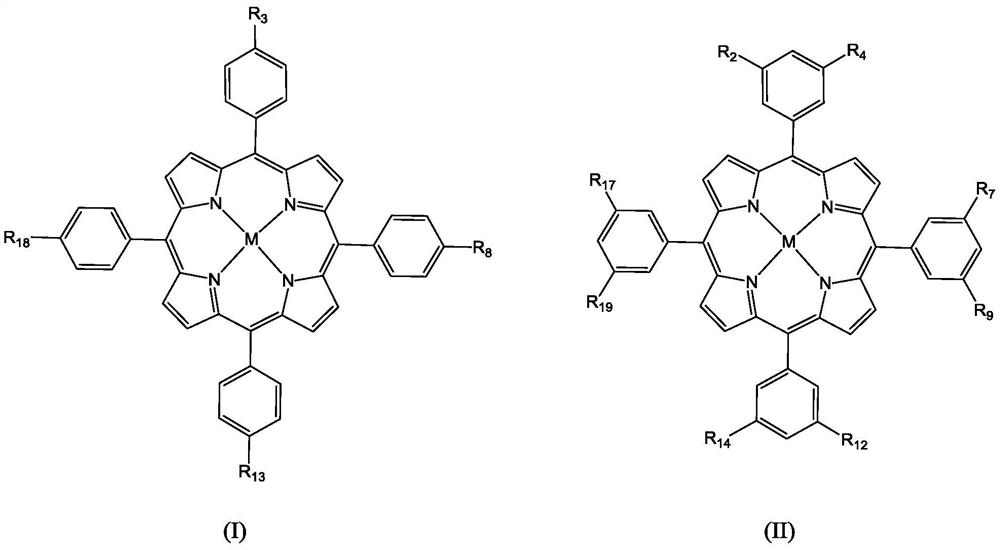
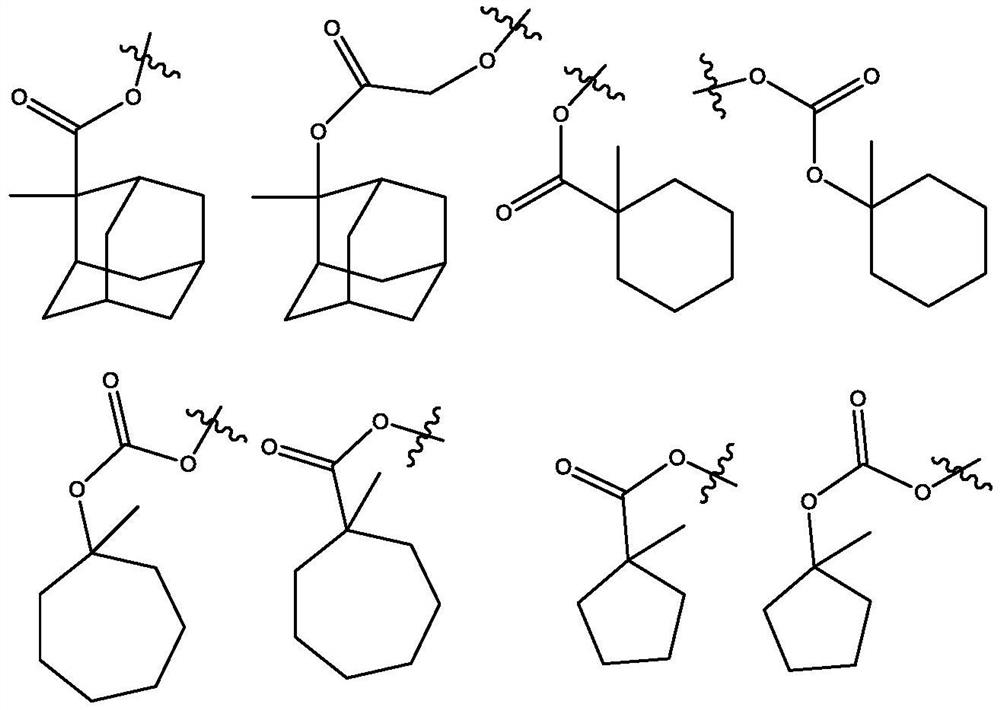
Molecular glass chemical amplification photoresist based on metalloporphyrin and preparation method and application thereof
A metalloporphyrin and metal salt technology, applied in the field of photoresist, can solve problems such as uneven distribution, limited lithographic resolution and line edge roughness, difficult to adapt to high-resolution lithography, etc., to meet the requirements of lithography technology required effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: Photoresist composition based on 5,10,15,20-tetrakis(4-tert-butylcarbonate phenyl) porphyrin zinc compound
[0068] Photoresist composition:
[0069] (1) Substrate: 5,10,15,20-tetrakis(4-tert-butylcarbonate phenyl)porphyrin zinc (compound 1-D) 50mg
[0070] (2) Photoacid generator: 2.5mg
[0071] (3) Organic base: trioctylamine 0.125mg
[0072] (4) Organic solvent: propylene glycol methyl ether acetate (PGMEA) 1.0ml
[0073] (5,10,15,20-tetrakis(4-tert-butylcarbonate phenyl)porphyrin zinc):
[0074]
[0075] Synthesis of Compound A:
[0076] Atmospheric pressure distillation of pyrrole, take 5 mL of distilled pure pyrrole, add 10 mL of propionic acid to dilute, set aside. Measure 4.5g of 4-methoxybenzaldehyde and 220mL of propionic acid into a 500mL three-necked bottle, stir rapidly, and heat to reflux; after the drug is completely dissolved, use a peristaltic pump to add the propionic acid solution of pyrrole drop by drop for about 30 minutes. Aft...
Embodiment 2
[0083] Example 2: Photoresist composition based on 5,10,15,20-tetrakis(4-tert-butylcarbonate phenyl) porphyrin zirconium compound
[0084] Photoresist composition:
[0085] (1) Substrate: 5,10,15,20-tetrakis(4-tert-butylcarbonate phenyl)porphyrin zirconium 50mg
[0086] (2) Photoacid generator: 2.5mg
[0087] (3) Organic base: trioctylamine 0.125mg
[0088] (4) Organic solvent: propylene glycol methyl ether acetate (PGMEA) 1.0ml
[0089] Wherein, 5,10,15,20-tetrakis(4-tert-butylcarbonate phenyl)porphyrin zirconium is prepared by the same method as compound 1-D in Example 1, except that anhydrous ZrCl 4 replace ZnCl 2 ·6H 2 O. HRMS(MALDI): theoretical value [M+H] + , 1167.3328; experimental value, 1167.3330.
Embodiment 3
[0090] Example 3: Photoresist composition based on 5,10,15,20-tetrakis(4-tert-butylcarbonate phenyl)porphyrin hafnium compound
[0091] Photoresist composition:
[0092] (1) Matrix: 50mg of hafnium 5,10,15,20-tetrakis(4-tert-butylcarbonate phenyl)porphyrin
[0093] (2) Photoacid Generator 2.5mg
[0094] (3) Organic base: trioctylamine 0.125mg
[0095] (4) Organic solvent: propylene glycol methyl ether acetate (PGMEA) 1.0ml
[0096] Among them, 5,10,15,20-tetrakis(4-tert-butylcarbonate phenyl) hafnium porphyrin is prepared by the same method as compound 1-D in Example 1, except that HfCl 4 Change ZnCl 2 ·6H 2 O. HRMS(MALDI): theoretical value [M+H] + , 1257.3736, experimental value, 1257.3738.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com