Crocetin solid dispersion and preparation method thereof
A technology of solid dispersion and crocetin, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., can solve problems such as toxic side effects, aging, and affecting drug solubilization effects , to achieve the effects of increased solubility, improved stability, and increased bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
[0048] table 5
[0049]
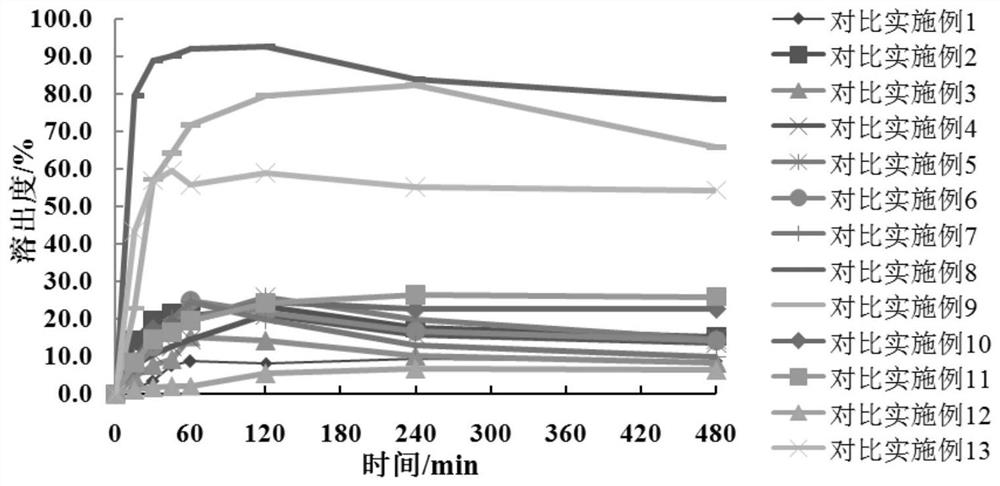
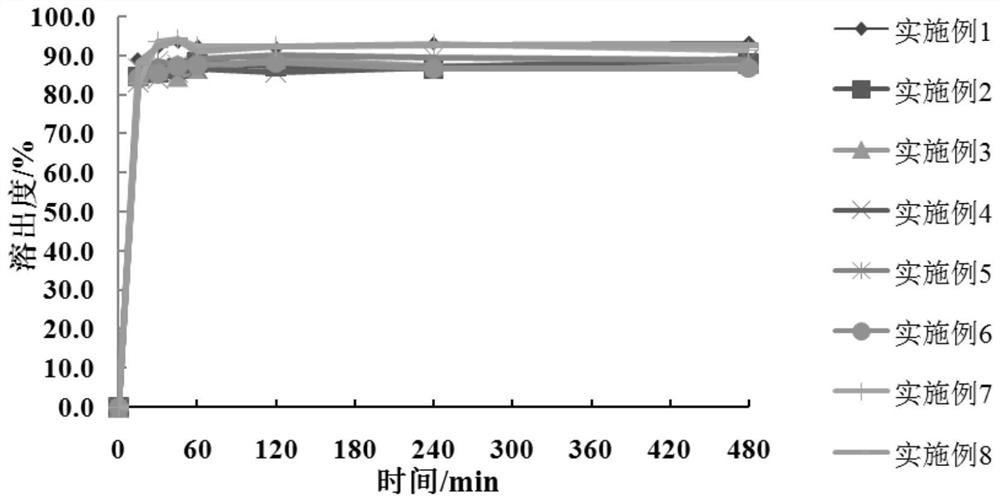
[0050] According to Table 5, the meglumine and the hydrophilic polymer carrier material in Examples 1-6 are dissolved in water and ultrasonicated for 1 h, so that the hydrophilic polymer material and meglumine are dissolved and cross-linked to form a carrier polymer I: Add crocetin to the system containing the carrier polymer I, and dissolve the crocetin by ultrasonication or stirring. The solvent is removed by rotary evaporation or spray drying to obtain a solid dispersion of crocetin, which is passed through a 100-mesh sieve. With pH 6.8 phosphate buffer as the dissolution medium, the volume of the dissolution medium is 900mL, and the rotation speed is 75rpm. The dissolution test is carried out by the paddle method, the dissolution results are determined by high performance liquid chromatography, and the cumulative dissolution rate is calculated.
[0051] Table 6
[0052]
[0053]
[0054] According to the dissolution rate obtained in Tab...
Embodiment 7
[0056] Crocetin solid dispersion, comprises the composition of following weight ratio:
[0057] Crocetin 10g
[0058] Soluplus 10g
[0059] Meglumine 40g
[0060] The preparation process is as follows:
[0061] (1) Soluplus and meglumine were dissolved in 500 mL of 95% ethanol solution and ultrasonicated for 1 h to obtain carrier polymer I.
[0062] (2) Adding crocetin to the solution system containing the carrier polymer I obtained in step (1), and ultrasonically dissolving the crocetin.
[0063] (3) Use a rotary evaporator to evaporate the solvent from the solution obtained in step (2), dry it under reduced pressure at 60° C. for 12 hours, collect the solid, pulverize it and pass it through a 100-mesh sieve to obtain the crocetin solid dispersion.
Embodiment 8
[0065] Crocetin solid dispersion, comprises the composition of following weight ratio:
[0066] Crocetin 10g
[0067] Soluplus 20g
[0068] Meglumine 40g
[0069] The preparation process is as follows:
[0070] Step 1) Soluplus and meglumine were dissolved in 500 mL of purified water, and stirred at 300 rpm for 1 h to obtain carrier polymer I.
[0071] Step 2) adding crocetin to the solution system containing carrier polymer I obtained in step 1), and stirring to dissolve the crocetin.
[0072] Step 3) Use the spray drying method to remove moisture from the solution obtained in step 2), the inlet air temperature is 135°C, the outlet air temperature is 60°C, and the air volume is 0.8m 3 / min, the atomization pressure is 8kPa, and the flow rate is 1.5mL / min. During the spray drying process, the temperature of the mixed solution containing the drug is kept at 70°C.
[0073] Step 4) The spray-dried product was dried under reduced pressure at 60° C. for 12 hours, the solid was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com