Method for determining related substances of ampicillin sodium and sulbactam sodium for injection
A technology for ampicillin sodium and sulbactam sodium and related substances is applied in the field of determination of related substances of ampicillin sodium and sulbactam sodium for injection, which can solve the problems of measurement interference, low purity, and many impurities, so as to avoid interference and improve the The effect of specificity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
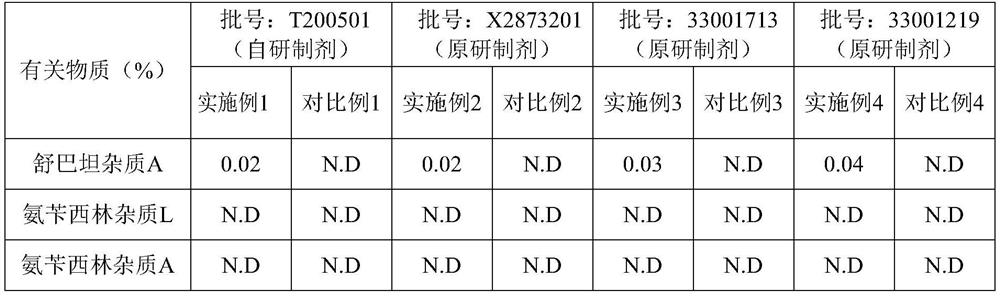
[0034] The batch number of sodium Sulbartan soda soda soda is used in injection is: T200501.
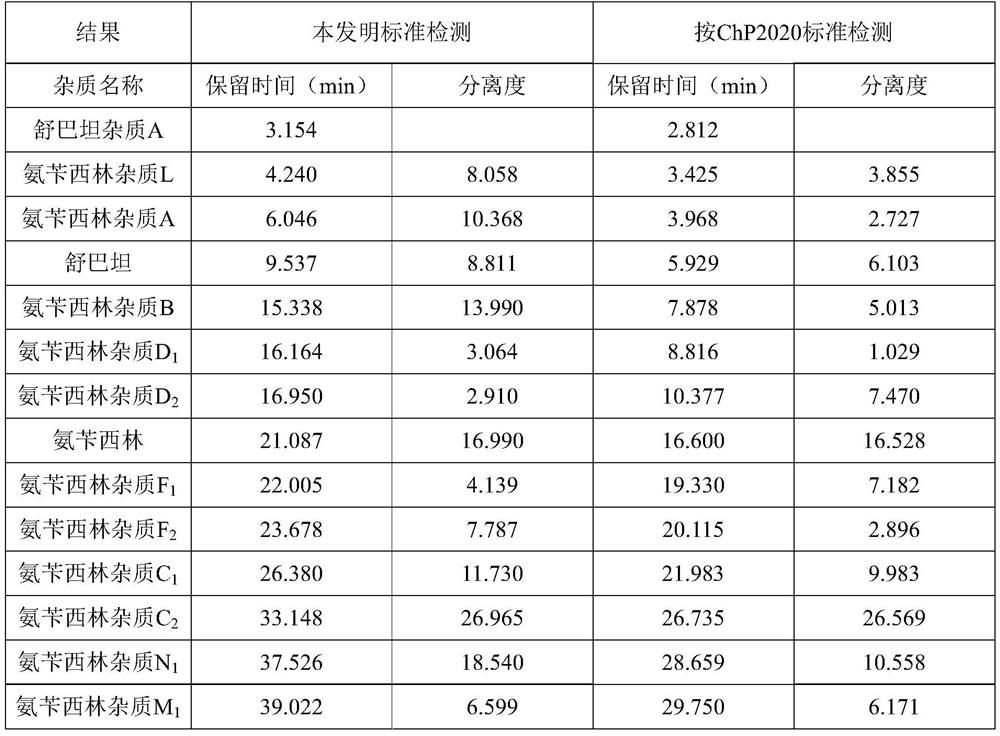
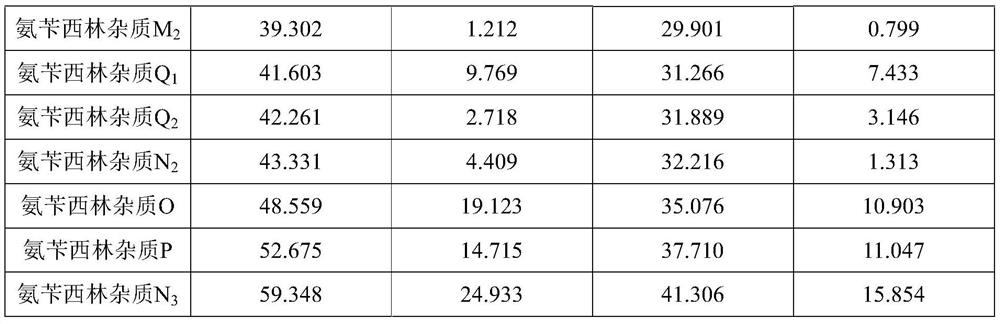
[0035] Detection method: High performance liquid chromatography, using the standard of the present invention, using octadecyl silicone bile silica gel as a filler (4.6 mM × 250 mM, 5 μm).
[0036] Mobile phase A: 0.02 mol / L of sodium phosphate (pH is adjusted to 3.50 ± 0.05) with a 1 mol / L phosphoric acid solution to 3.50 ± 0.05); mobile phase B: acetonitrile.
[0037] Detection wavelength: 230 nm; column temperature: 40 ° C; flow rate: 1.0mL / min calculation method: 1% self-control method in size: 10 μL ...; linear elution gradient as shown in Table:
[0038] Mobile phase gradient
[0039] T (min) A(%) B(%) 0 98 2 5 93 7 10 93 7 60 60 40 70 60 40 71 98 2 80 98 2
Embodiment 2
[0041] Example 2 is consistent with the detection method of Example 1, and the difference is that the batch number of sodium Sulbawa sodium Sulbawa in injection is: x2873201.
[0042] Detection method: High performance liquid chromatography, using the standard of the present invention, using octadecyl silicone bile silica gel as a filler (4.6 mM × 250 mM, 5 μm).
[0043] Mobile phase A: 0.02 mol / L of sodium phosphate (pH is adjusted to 3.50 ± 0.05) with a 1 mol / L phosphoric acid solution to 3.50 ± 0.05); mobile phase B: acetonitrile.
[0044] Detection wavelength: 230 nm; column temperature: 40 ° C; flow rate: 1.0mL / min calculation method: 1% self-control method in size: 10 μL ...; linear elution gradient as shown in Table:
[0045] Mobile phase gradient
[0046] T (min) A(%) B(%) 0 98 2 5 93 7 10 93 7 60 60 40 70 60 40 71 98 2 80 98 2
Embodiment 3
[0048] Example 3 was consistent with the detection method of Example 1, and the difference is that the batch number of ampicillin sodium Sulbawa soda is used as: 33001713.
[0049] Detection method: High performance liquid chromatography, using the standard of the present invention, using octadecyl silicone bile silica gel as a filler (4.6 mM × 250 mM, 5 μm).
[0050] Mobile phase A: 0.02 mol / L of sodium phosphate (pH is adjusted to 3.50 ± 0.05) with a 1 mol / L phosphoric acid solution to 3.50 ± 0.05); mobile phase B: acetonitrile.
[0051] Detection wavelength: 230 nm; column temperature: 40 ° C; flow rate: 1.0mL / min calculation method: 1% self-control method in size: 10 μL ...; linear elution gradient as shown in Table:
[0052] Mobile phase gradient
[0053] T (min) A(%) B(%) 0 98 2 5 93 7 10 93 7 60 60 40 70 60 40 71 98 2 80 98 2
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com