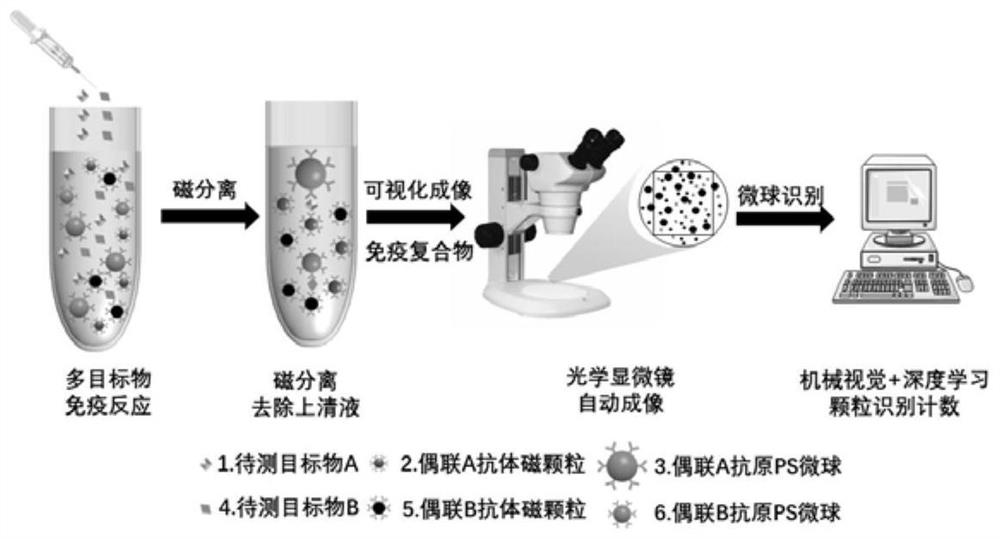
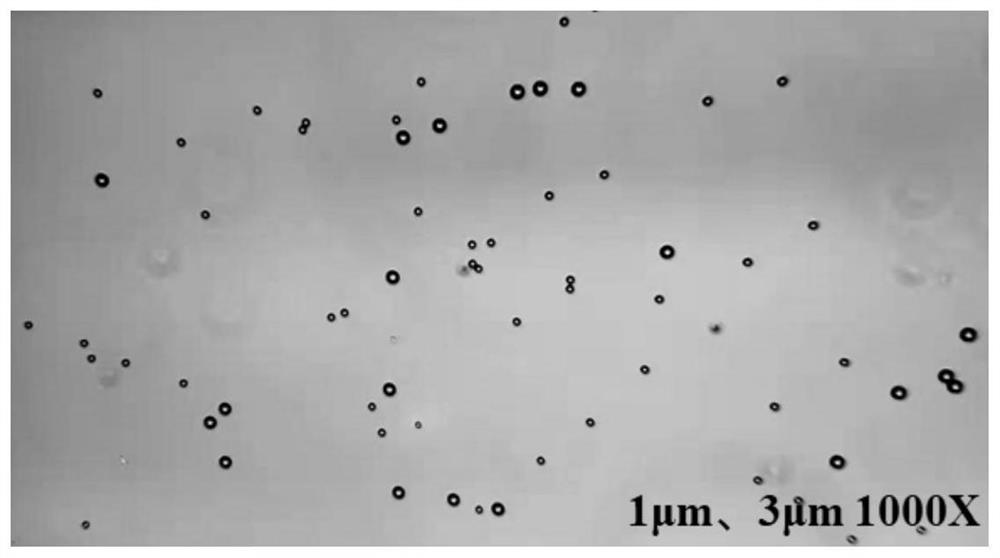
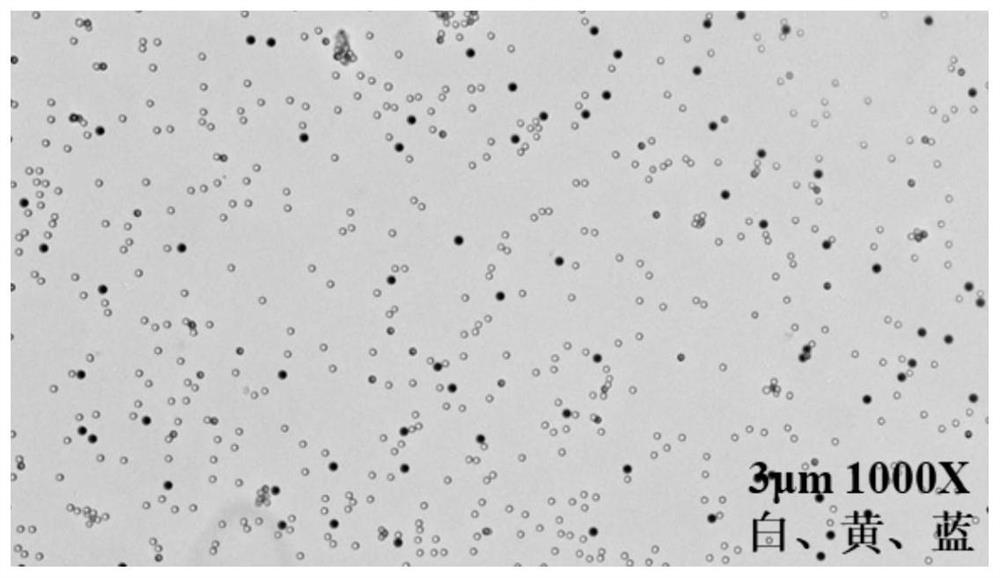
A Biochemical Analysis Method Based on Magnetic Separation for Simultaneous Detection of Multiple Targets
A technology for biochemical analysis and target objects, applied in the field of biochemical analysis, can solve problems such as cumulative errors, affecting the stability and accuracy of detection results, and complicated operations, and achieves strong capabilities, improved detection sensitivity and accuracy, and fewer operating steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Quantitative detection of procalcitonin (PCT) in whole blood
[0048] (1) Preparation of nano-magnetic particle-procalcitonin capture antibody conjugate and polystyrene microsphere-procalcitonin detection antibody conjugate
[0049] Take 2 mg of carboxyl-modified magnetic nanoparticles (200 nm, 10 mg / mL), wash twice with MES buffer (pH=6.0), resuspend in MES buffer and add 50 μL EDC (10 mg / mL) and 25 μL NHS (10 mg / mL) mL), mixed and activated at room temperature for 15 min. After activation, wash twice with PBS buffer, resuspend in PBS buffer, add 0.2 mg of procalcitonin (PCT) to capture the antibody, and react at room temperature for 2-4 hours; after the reaction, block with 1% BSA solution for 30 minutes, After the blocking was completed, the cells were washed twice with PBST buffer, and the nano-magnetic particle-procalcitonin capture antibody conjugate was resuspended in PBS buffer and stored at 4°C for later use.
[0050] Take 2 mg of carboxyl-modified ...
Embodiment 2
[0068] Embodiment 2 Quantitative detection of antibiotic molecules chloramphenicol, amoxicillin and neomycin
[0069] (1) Preparation of nanomagnetic particles-antibody conjugates of different kinds of antibiotics and polystyrene microspheres of different particle sizes-complete antigen conjugates of different kinds of antibiotics
[0070] Take 3 mg of carboxyl-modified magnetic nanoparticles (200 nm, 10 mg / mL) into 3 parts, each 1 mg, wash twice with MES buffer (pH=6.0), and add 20 μL of MES buffer after resuspending each part. EDC (10 mg / mL) and 10 μL NHS (10 mg / mL) were activated at room temperature for 15 min and mixed well. After activation, they were washed twice with PBS buffer, resuspended in PBS buffer, and then added 0.1 mg of chloramphenicol antibody, 0.1 mg of amoxicillin antibody and 0.1 mg of neomycin antibody, respectively, and reacted at room temperature for 2-4 hours; After the reaction, the cells were blocked with 1% BSA solution for 30 min, washed twice wit...
Embodiment 3
[0082] Example 3 Quantitative detection of C-reactive protein (CRP), procalcitonin (PCT) and interleukin-6 (IL-6) in whole blood
[0083] (1) Preparation of nano-magnetic particles-different kinds of biomarkers capture antibody conjugates and polystyrene microspheres of different colors-different kinds of biomarkers detection antibody conjugates
[0084]Take 3 mg of carboxyl-modified magnetic nanoparticles (200 nm, 10 mg / mL) into 3 parts, each 1 mg, wash twice with MES buffer (pH=6.0), and add 20 μL of MES buffer after resuspending each part. EDC (10 mg / mL) and 10 μL NHS (10 mg / mL) were activated at room temperature for 15 min and mixed well. After activation, wash twice with PBS buffer, resuspend in PBS buffer, add 0.1 mg of CRP capture antibody, 0.1 mg of PCT capture antibody, and 0.1 mg of IL-6 capture antibody, respectively, and react at room temperature for 2-4 hours; the reaction ends After blocking with 1% BSA solution for 30 min, washed twice with PBST buffer after bl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com