Preparation method and application of organic metal compound
A technology of organometallic compounds, which is applied in the field of preparation of organometallic compounds, can solve the problems of high experimental conditions, impractical application, and difficult batch production, and achieve high conversion rate, excellent thermal stability, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation of embodiment 1 carbazole sodium
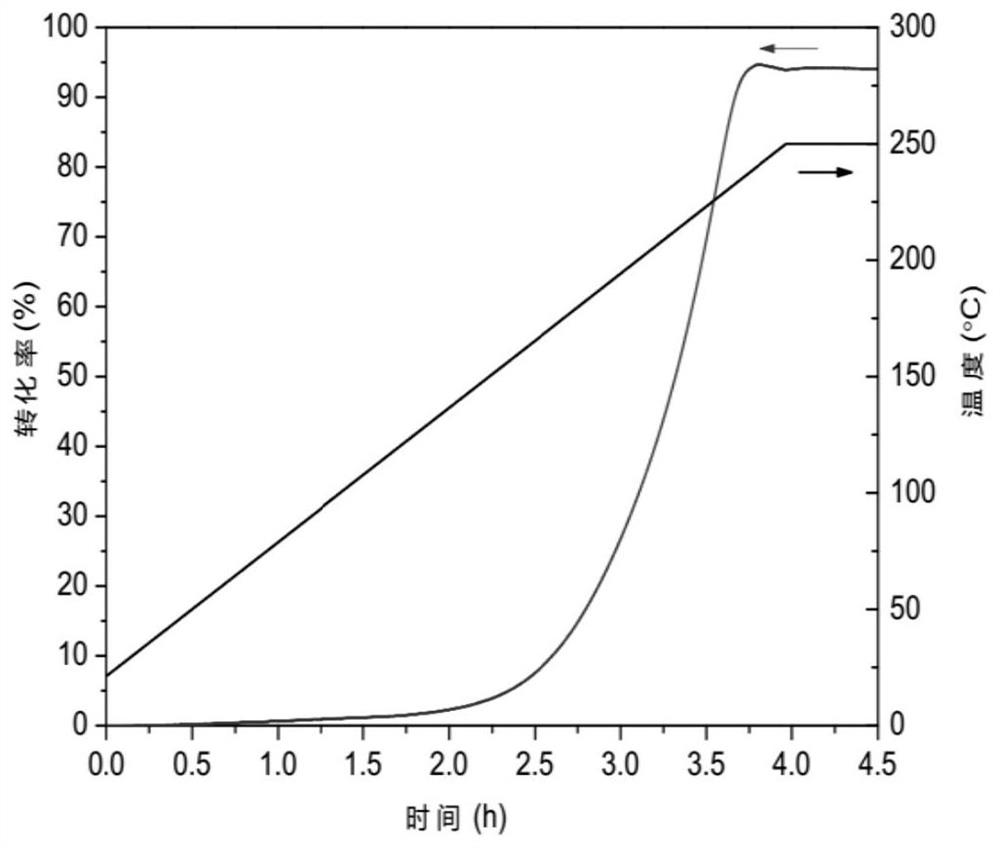
[0047] In the glove box, under the protection of argon atmosphere, accurately weigh 1.7601g of carbazole powder, put it into a ball mill jar, and accurately weigh 0.2664g of sodium hydride powder, put it into the same ball mill jar, and mix the above two The powders were preliminarily mixed, the ball mill jar was sealed, and put into the ball mill. At room temperature, the ball mill speed was set to 150 rpm, and the ball mill time was set to 4 hours. Put the ball-milled product in a stainless steel iron tank, seal and heat, set the temperature at 250°C, and keep the temperature constant until the air pressure no longer changes. The reaction conversion rate can be calculated by detecting the pressure change in the stainless steel tube. When the pressure change reaches the theoretical amount of dehydrogenation, the reaction is complete. figure 1 Prepare the conversion rate curve of carbazole sodium for the ball milling m...
Embodiment 2
[0048] The measurement of embodiment 2 carbazole sodium conductivity
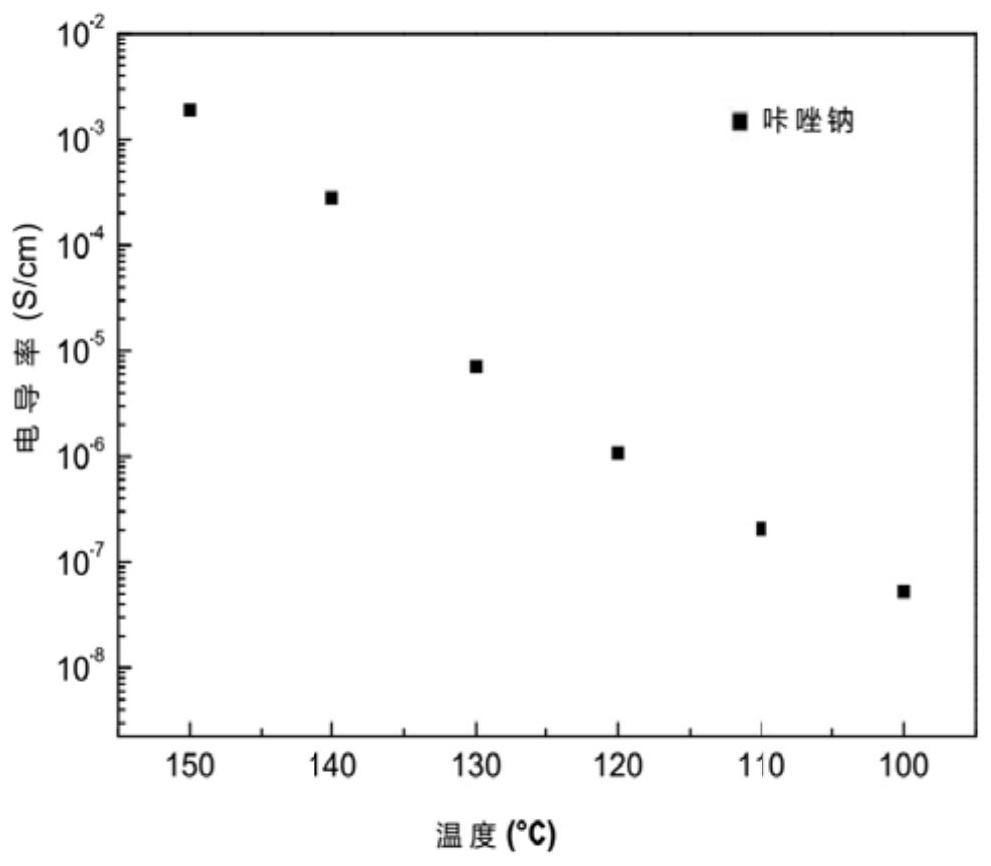
[0049]Take the above-mentioned appropriate amount of carbazole sodium, put it in a mold with a diameter of 15mm, oscillate and distribute it evenly, apply static pressure to form the powder, and press it into a disc with a thickness of 1.00mm, and then place the disc on two equal-diameter In the middle of the metal lithium sheet, a "sandwich" structure is formed, and the "sandwich" structure is wrapped with a commercial battery case, and pressure is applied to seal the battery case. Connect the battery assembled above to the electrochemical workstation. After the open circuit voltage is stable, start the measurement. Set the temperature to 100, 110, 120, 130, 140, and 150°C in sequence, and keep the temperature for 2 hours before each temperature measurement to make the battery in a steady state. image 3 is the conductivity diagram of carbazole sodium prepared by ball milling method, by image 3 It can b...
Embodiment 3
[0050] The preparation of embodiment 3 indolelithium
[0051] In the glove box, under the protection of argon atmosphere, accurately weigh 1.1820g of indole powder, put it into a ball mill jar, and accurately weigh 0.0795g of lithium hydride powder, put it into the same ball mill jar, and mix the above two The powders are preliminarily mixed, the ball mill jar is sealed, and put into a ball mill. At room temperature, the ball mill speed is set to 150 revolutions per minute, and the ball mill time is set to 4 hours. The reaction conversion rate can be calculated by detecting the pressure change in the ball mill tank. When the pressure change reaches the theoretical dehydrogenation amount, it indicates that the product has been synthesized. Figure 4 Indolithium and indole prepared by ball milling 1 H-NMR spectrum. By comparing indolelithium and indole 1 From the H-NMR spectrum, it can be seen that the original -NH peak in indole has disappeared, and other peak positions hav...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com