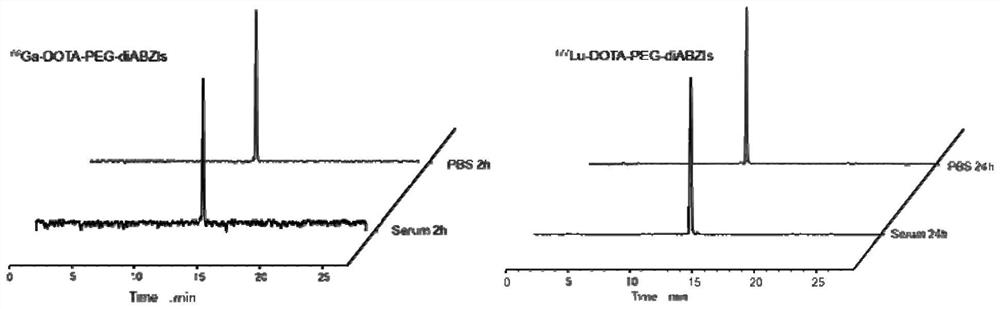
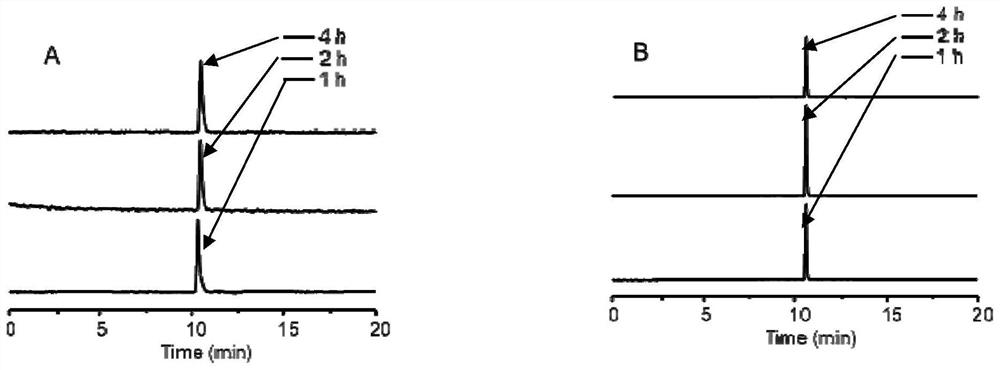
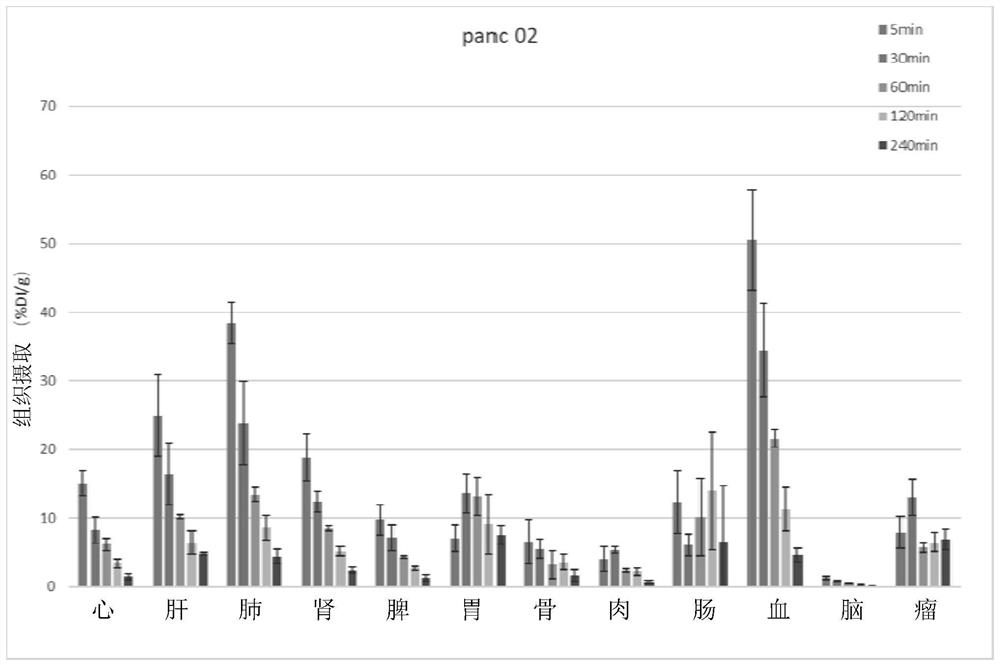
A kind of interferon-stimulated protein targeting compound, its radiolabel, and their preparation method and application
A compound and interferon technology, which is applied in the field of radiopharmaceuticals and medical imaging, achieves the effect of simple preparation method, strong labeling ability, and broadened application scenarios
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1. Dimeric amidobenzimidazole compound——DABI-PEG 2 -NH 2 (compound 12-1)
[0081] The synthetic route is as follows:
[0082]
[0083] Specifically include the following steps:
[0084] 1) Synthesis of compound 3
[0085] With 4-chloro-3 nitro-5 methoxybenzamide (compound 1) and (4-aminobut-2-en-1-yl) tert-butyl carbamate (compound 2) according to 1:1.2 molar ratio Dissolve in an appropriate amount of isopropanol, then add DIPEA in an equimolar ratio to compound 1 dropwise into the reaction bottle, stir and react overnight at room temperature, carry out aromatic nucleophilic substitution reaction, monitor the reaction process by TLC, spin the solvent after the reaction, and pass the crude product through Silica gel column was purified to obtain compound 3.
[0086] 2) Synthesis of Compound 4
[0087] 1) Dissolve the obtained compound 3 with an appropriate amount of methanol, transfer it to the reaction flask, and then slowly add 50 mL of hydrochloric ac...
Embodiment 2
[0098] Example 2. Dimeric amidobenzimidazole compound——DABI-Et 3 -NH 2 (compound 12-2)
[0099] The synthetic route is as follows:
[0100]
[0101] Specifically include the following steps:
[0102] 1) Synthesis of compound 3
[0103] Operation steps are the same as step 1) of embodiment 1.
[0104] 2) Synthesis of Compound 4
[0105] Operation steps are the same as step 2) of embodiment 1.
[0106] 3) Synthesis of compound 7-2
[0107] Weigh an equimolar amount of compound 5 (4-chloro-3 nitro-5 hydroxybenzamide) and compound 6-2 (6-(N-tert-butoxycarbonylamino)-1-hexanol, i.e. HO-Et 3 -NH-Boc) and triphenylphosphine (PPh 3 ) was dissolved in THF, and 2 times the molar amount of diisopropyl azodicarboxylate (DIAD) was added under ice-bath conditions to carry out a bimolecular nucleophilic substitution reaction, and reacted at room temperature for 24 hours. After the reaction was completed, the solvent was removed, and the crude product was passed through Compound 7...
Embodiment 3
[0117] Example 3. Interferon-stimulated protein-targeted radionuclide-labeled compound——[ 18 F]F-NOTA-PEG 2 -DABI
[0118] The synthetic route is as follows:
[0119]
[0120] Specifically include the following steps:
[0121] 1) NOTA-PEG 2 - Synthesis of DABI
[0122] Weigh the DABI-PEG prepared in Example 1 2 -NH 2 Dissolve in appropriate amount of DMF in equimolar ratio with NOTA-NHS, then add triethylamine, react for 48h, after reaction, add appropriate amount of hydrochloric acid to acidify and purify with high performance liquid chromatography (HPLC), mobile phase conditions: semi-preparative chromatographic column (250 ×10mm, 5μm, Thermo), phase A: water + 0.1% trifluoroacetic acid (TFA), phase B: acetonitrile + 0.1% TFA. Gradient elution conditions: 0-5min: 20%-28% B phase; 5-15min: 28% B phase; 15-20 min: 28%-39% B; 20-25min: 39%-95% B; 25 -40min: 20% phase B, flow rate: 3mL / min, UV wavelength: 254nm. The purified solution was dried in vacuo to obtain the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com