Thin-layer chromatography identification method of Danwei Kang capsule
A technology of thin-layer chromatography and Danweikang, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., to achieve the effects of shortening the preparation time, good separation effect and controlling the quality of medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] (1) Preparation of the test solution:
[0047] Take 3g of the content of "Danweikang Capsule" and add 45mL of methanol to ultrasonic treatment for 30 minutes, filter, evaporate the filtrate to dryness, add 3mL of methanol to dissolve the residue as the test solution;
[0048] (2) Thin-layer chromatographic identification of swertiamarin:
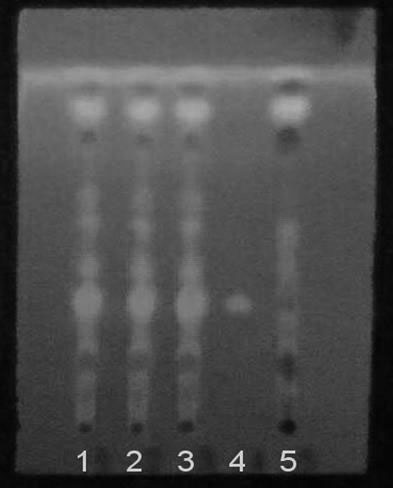
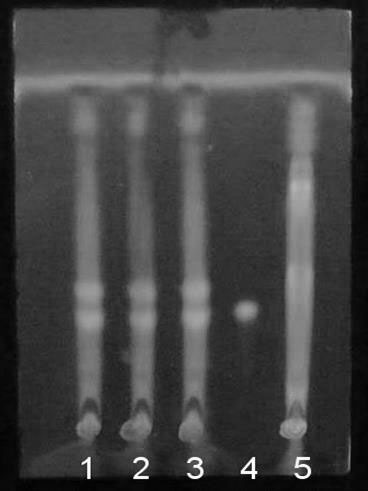
[0049] Dissolve the swertomacroside reference substance in methanol to make a 0.5 mg / mL reference substance solution; respectively draw 3 μL of the test solution and the reference solution, and place them on the same thin-layer chromatographic plate, and use chloroform-methanol -Water-formic acid solution is the developing agent for upward development, take it out, and dry it; spray the thin-layer chromatography with sulfuric acid-ethanol solution, and check it under the ultraviolet light (254nm) after drying; Blue-green fluorescent spots of the same hue appear on the corresponding position of the chromatogram of the reference substa...
Embodiment 1
[0114] Example 1: TLC Identification of Swertamarin in "Danweikang Capsules"
[0115] Take 3g of the content of "Danweikang Capsule", add 45mL of methanol, ultrasonically treat for 30 minutes, filter, evaporate the filtrate to dryness, add 3mL of methanol to dissolve the residue, and use it as the test solution. Take swertamarin reference substance and dissolve it in methanol to make a 0.5mg / mL reference substance solution.
[0116] In addition, 9 medicinal materials in the prescription of "Danweikang Capsules" except Qingye Gallbladder were taken, and a negative control sample without Qingye Gallbladder was made according to the preparation method stipulated in the standard of "Danweikang Capsules", and prepared according to the above method. Become a negative control solution lacking Folium bile.
[0117] Draw 3 μL each of the test solution, reference solution, and negative control solution, respectively, and place them on the same silica gel G thin-layer chromatography pla...
Embodiment 2
[0120] Example 2: TLC Identification of Wogonin in "Danweikang Capsules"
[0121] Take 3g of the content of "Danweikang Capsule", add 45mL of methanol, ultrasonically treat for 30 minutes, filter, evaporate the filtrate to dryness, add 3mL of methanol to dissolve the residue, and use it as the test solution. The wogonin reference substance was dissolved in methanol to prepare a 0.3mg / mL reference substance solution.
[0122] In addition, 9 medicinal materials of the prescription "Danweikang Capsules" except Southwest Scutellariae were taken, and a negative control sample without Southwest Scutellariae was made according to the preparation method stipulated in the standard of "Danweikang Capsules", and the lack of Southwest Scutellariae was prepared according to the above method. Negative control solution of Scutellaria baicalensis.
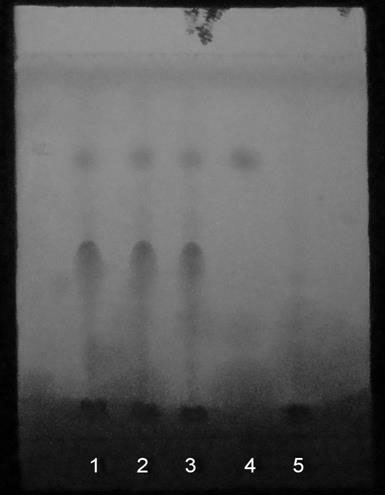
[0123] Draw 3 μL each of the test solution, reference solution, and negative control solution, respectively, and place them on the same silica gel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com