Method for preparing monophenol chemicals by catalyzing lignin depolymerization through metal organic framework material derivative loaded ruthenium
A metal-organic framework and derivative technology, which is used in the field of biomass degradation, metal-organic framework material derivatives supported ruthenium catalyzed lignin conversion to prepare monophenolic chemicals, and can solve the problems of catalyst performance degradation, catalyst susceptibility to deactivation, etc. , to achieve the effect of high conversion rate, lignin conversion rate and single-classification chemical yield improvement, and simple process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
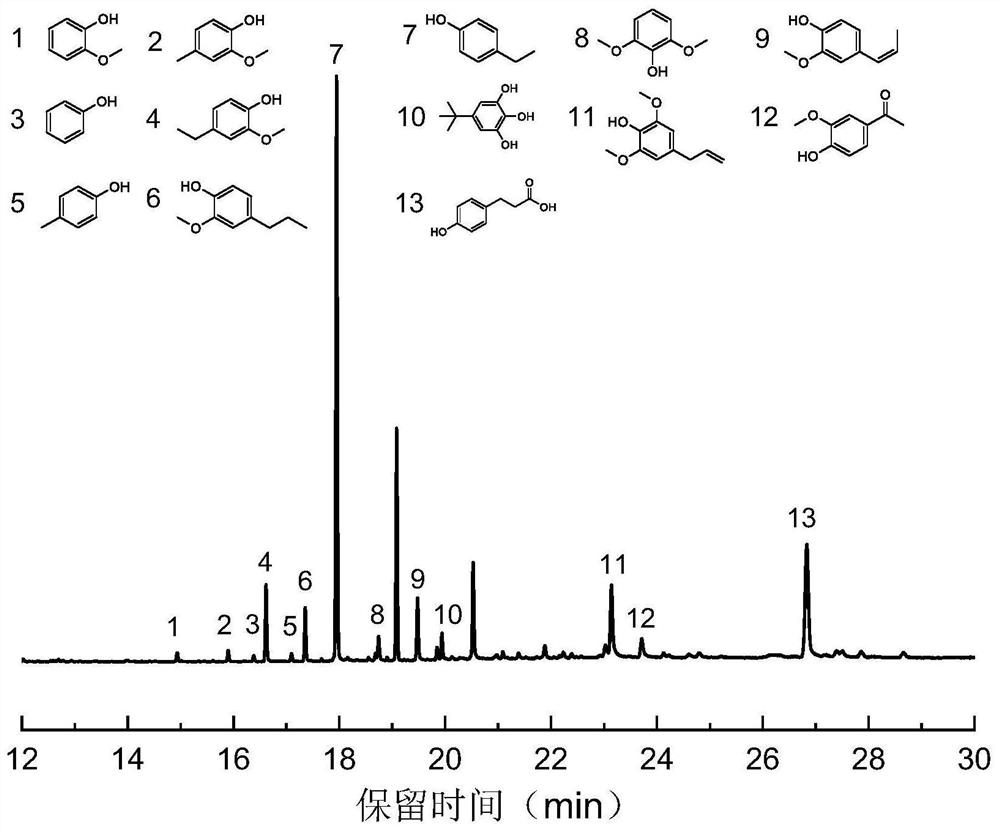
Examples
Embodiment 1
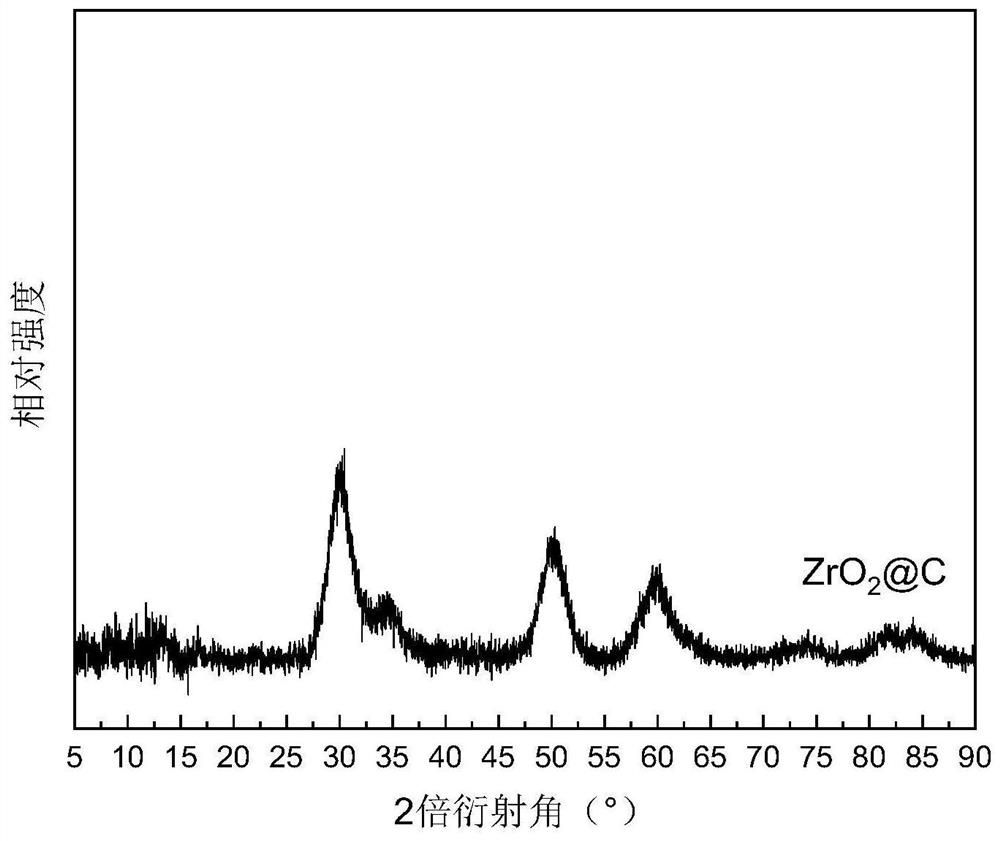
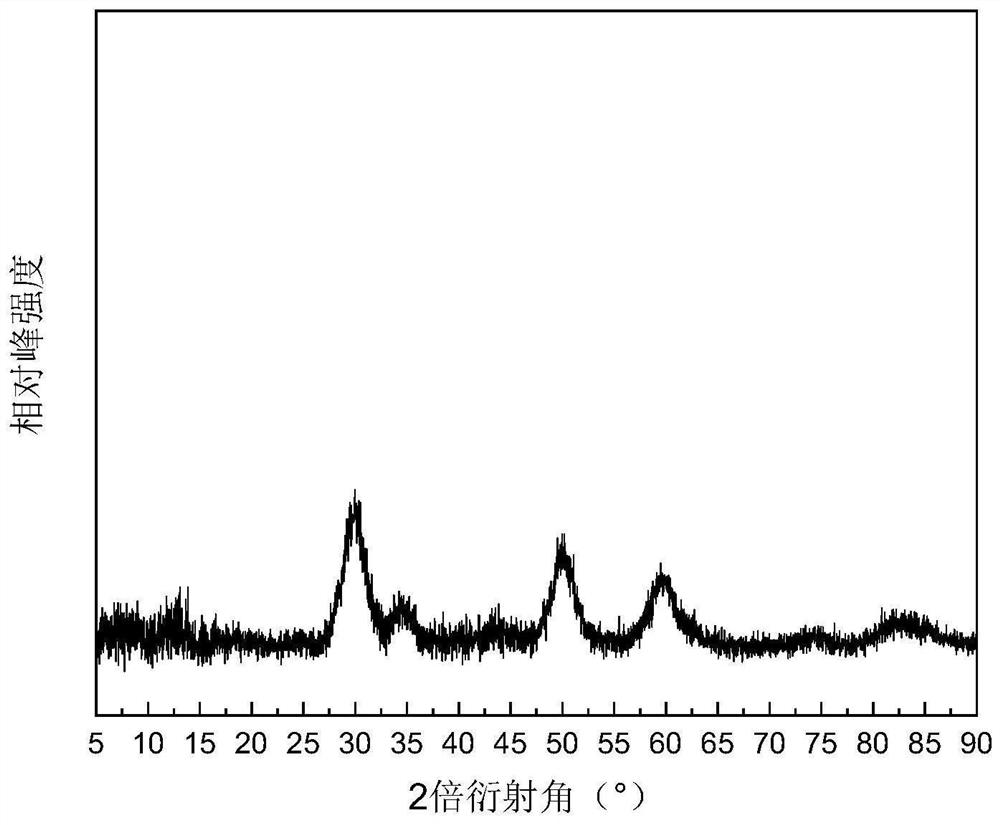
[0035] Example 1: 3wt.%Ru / ZrO 2 Preparation of @C catalyst
[0036] Zirconium-based metal-organic framework derivatives supported ruthenium-based metal catalysts through a two-step process:
[0037] (1) ZrO 2 Preparation of @C carrier: Weigh 1.16g of ZrCl with a purity of 99.9% 4 Solid, 0.83g of PTA (terephthalic acid) solid with a purity of 99%, the two were added to 250mL of a DMF solution with a purity of 99.5%, and 25mL of acetic acid with a purity of 99.5% was added. The prepared reaction liquid system was ultrasonicated for 30 min, transferred to a reaction vessel, and reacted at 120° C. for 24 h. After the reaction was completed, the reaction system was cooled to room temperature and centrifuged to obtain a white solid. The obtained white solid was washed several times with DMF, ethanol, and deionized water respectively, dried overnight in a vacuum oven, and ground to obtain a white powder, which is the carrier precursor. Transfer the white powder to a tube furnace...
Embodiment 2
[0040] Example 2: 3wt.%Ru / ZrO 2 Preparation of @C catalyst
[0041] Zirconium-based metal-organic framework derivatives supported ruthenium-based metal catalysts through a two-step process:
[0042] (1) ZrO 2 Preparation of @C carrier: Weigh 1.16g of ZrCl with a purity of 99.9% 4 Solid, 0.83g of PTA solid with a purity of 99%, the two were added to 250mL of a DMF solution with a purity of 99.5%, and 25mL of acetic acid with a purity of 99.5% was added. The prepared reaction liquid system was ultrasonicated for 30 min, transferred to a reaction vessel, and reacted at 120° C. for 24 h. After the reaction was completed, the reaction system was cooled to room temperature and centrifuged to obtain a white solid. The obtained white solid was washed several times with DMF, ethanol, and deionized water respectively, dried overnight in a vacuum oven, and ground to obtain a white powder, which is the carrier precursor. Transfer the white powder to a tube furnace and calcinate at 55...
Embodiment 3
[0044] Example 3: 0.5wt.%Ru / ZrO 2 Preparation of @C catalyst
[0045] Zirconium-based metal-organic framework derivatives supported ruthenium-based metal catalysts through a two-step process:
[0046] (1) ZrO 2 Preparation of @C carrier: Weigh 1.16g of ZrCl with a purity of 99.9% 4 Solid, 0.83g of PTA solid with a purity of 99%, the two were added to 250mL of a DMF solution with a purity of 99.5%, and 25mL of acetic acid with a purity of 99.5% was added. The prepared reaction liquid system was ultrasonicated for 30 min, transferred to a reaction vessel, and reacted at 120° C. for 24 h. After the reaction was completed, the reaction system was cooled to room temperature and centrifuged to obtain a white solid. The obtained white solid was washed several times with DMF, ethanol, and deionized water respectively, dried overnight in a vacuum oven, and ground to obtain a white powder, which is the carrier precursor. Transfer the white powder to a tube furnace and calcinate at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com