Compound containing nitrate functional group and synthesis method of compound
A synthesis method and technology of nitrate esters, which are applied in the field of compounds containing nitrate ester functional groups and their synthesis, can solve problems such as complex reaction steps, and achieve the effects of easy installation and removal, avoiding pre-activation, and wide applicability of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
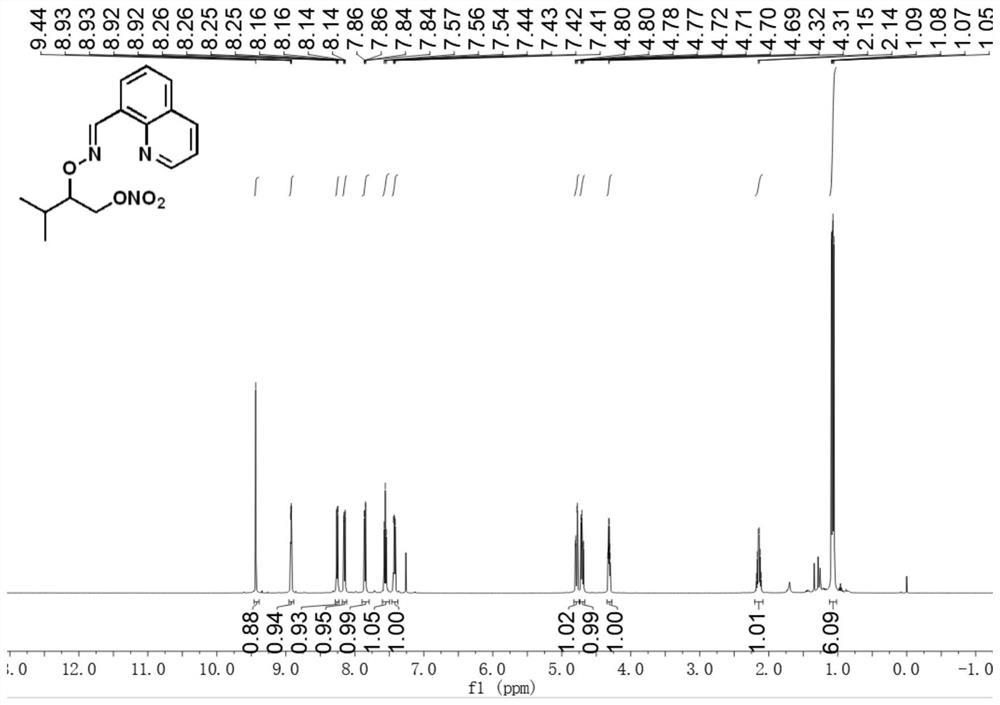
[0030] E)-3-Methyl-2-(((quinoline-8-methylene)amino)oxy)butyl nitrate
[0031] Accurately weigh E)-quinoline formaldehyde-(3-methylbutane-2-yl)oxime (24.2mg, 0.1mmol), palladium acetate (2.2mg, 0.01mmol), silver nitrite (46.2mg, 0.3mmol), selective fluorine reagent (53.1mg, 0.15mmol), tetrabutylammonium bisulfate (33.9mg, 0.1mmol) were transferred to the reaction vessel, 1.5ml 1,2-dichloroethane was added, and the bottle was screwed Plug, react at 80°C for 18h. After the reaction, the reaction solution was cooled to room temperature, filtered through short silica gel to remove insoluble impurities, extracted 3 times with ethyl acetate and water, and extracted 1 time with brine, and the organic phase was removed from the solvent to obtain a crude product, which was separated by column chromatography ( Eluent: ethyl acetate / petroleum ether=1:10) to obtain a pure and dry product with a yield of 72%. 1 H NMR (500MHz, CDCl 3 )δ9.44(s,1H),8.92(dd,J=4.1,1.7Hz,1H),8.26(dd,J=7.3,0.8...
Embodiment 2
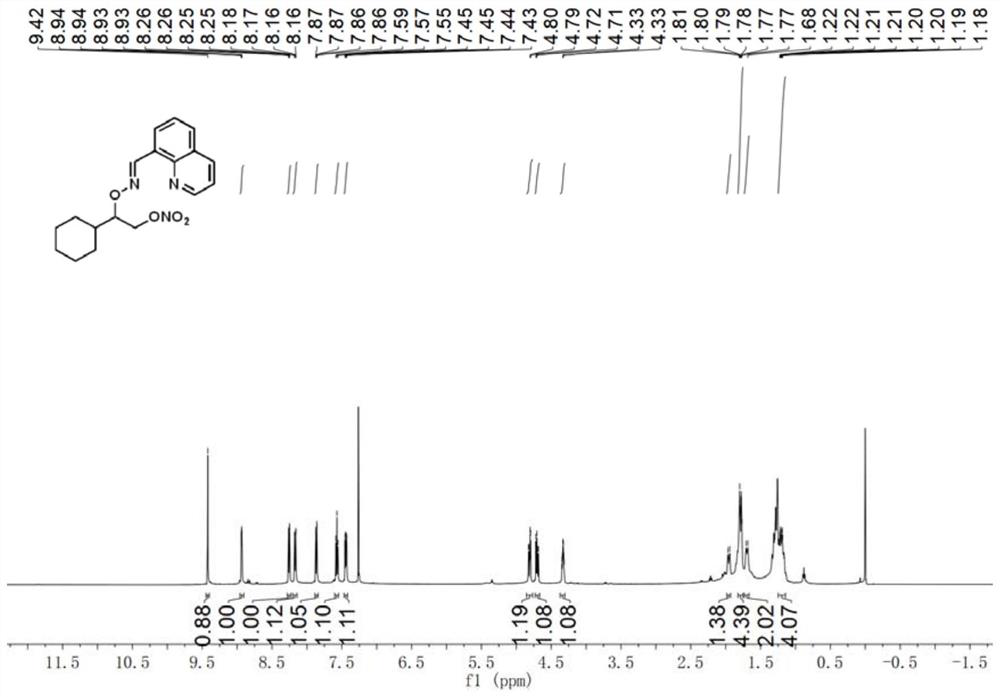
[0033] E)-2-cyclohexyl-2-(((quinoline-8-methylene)amino)oxy)ethyl nitrate
[0034] Accurately weigh E)-quinoline-8-formaldehyde O-(1-cyclohexylethyl)oxime (28.2mg, 0.1mmol), palladium acetate (2.2mg, 0.01mmol), silver nitrite (46.2mg, 0.3mmol ), selective fluorine reagent (53.1mg, 0.15mmol), tetrabutylammonium bisulfate (33.9mg, 0.1mmol) were transferred to the reaction vessel, 1.5ml 1,2-dichloroethane was added, and the stopper was tightened. Reaction at 80°C for 18h. After the reaction, the reaction solution was cooled to room temperature, filtered through short silica gel to remove insoluble impurities, extracted 3 times with ethyl acetate and water, and extracted 1 time with brine, and the organic phase was removed from the solvent to obtain a crude product, which was separated by column chromatography ( Eluent: ethyl acetate / petroleum ether=1:10), a pure and dry product was obtained with a yield of 63%. 1H NMR (500MHz, Chloroform-d) δ9.42(s, 1H), 8.94(dd, J=4.1, 1.8Hz, ...
Embodiment 3
[0036] E)-4-Phenyl-2-(((quinoline-8-methylene)amino)oxy)butyl nitrate
[0037] Accurately weigh E)-quinoline-8-carbaldehyde O-(4-phenylbutane-2-yl)oxime (30.4mg, 0.1mmol), palladium acetate (2.2mg, 0.01mmol), silver nitrite ( 46.2mg, 0.3mmol), selective fluorine reagent (53.1mg, 0.15mmol), tetrabutylammonium bisulfate (33.9mg, 0.1mmol) were transferred to the reaction vessel, 1.5ml 1,2-dichloroethane was added, Tighten the cork and react at 80°C for 18h. After the reaction, the reaction solution was cooled to room temperature, filtered through short silica gel to remove insoluble impurities, extracted 3 times with ethyl acetate and water, and extracted 1 time with brine, and the organic phase was removed from the solvent to obtain a crude product, which was separated by column chromatography ( Eluent: ethyl acetate / petroleum ether=1:10), a pure and dry product was obtained with a yield of 67%. 1 H NMR (500MHz, CDCl 3 )δ9.50(s,1H),8.95(dd,J=4.1,1.7Hz,1H),8.31–8.24(m,1H),8.17...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com