Fluorescent probe for selectively detecting H2S based on BODIPY dye targeted lysosome as well as preparation and application of fluorescent probe
A fluorescent probe and lysosome technology, applied in the field of fluorescent probes, can solve the problems of short detection fluorescence wavelength and long response time, and achieve the effects of convenient operation, simple synthesis method and obvious detection signal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
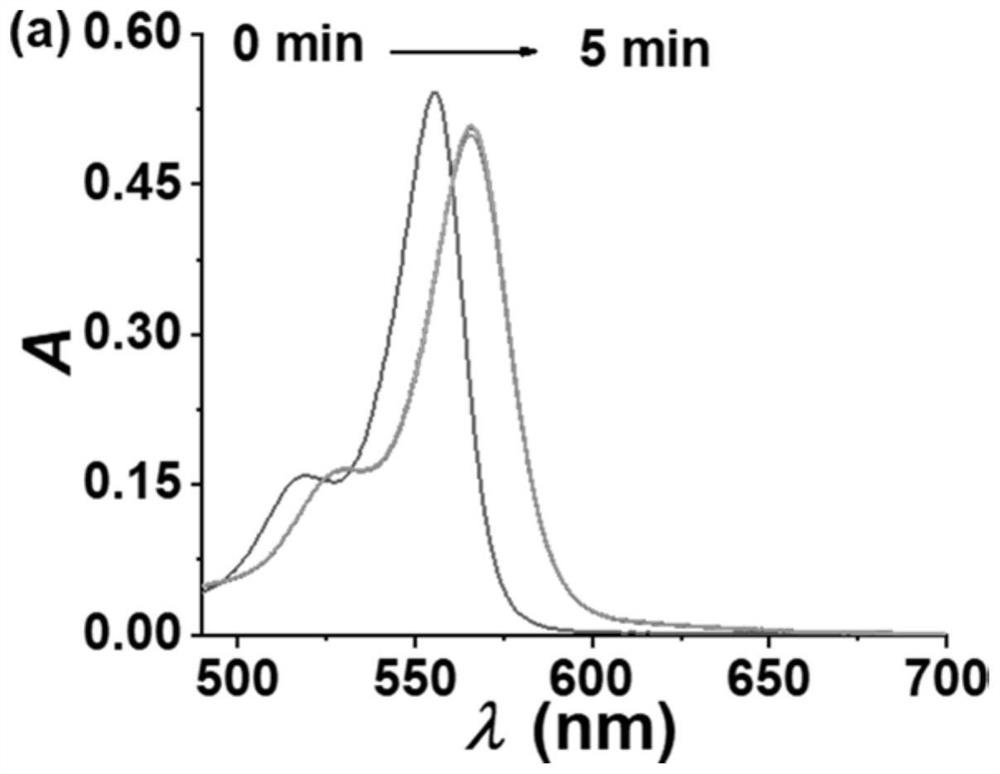
Image
Examples
Embodiment 1
[0037] The preparation steps of the probe are as follows:
[0038] (1) Preparation of Compound 1
[0039]
[0040] N 2 Under the protective ice-water bath, add 1.1 equivalents of triphenylphosphine into the tetrahydrofuran solvent to obtain a mixed solvent, dissolve 2-morpholine ethanol and p-hydroxybenzaldehyde in the mixed solvent according to the equivalent ratio of 1:1.2, and then add 1.1 An equivalent amount of diisopropyl azodicarboxylate was reacted at room temperature for 15 h, the solvent was removed under reduced pressure, and compound 1 was obtained by column chromatography;
[0041] 1 H NMR (400 MHz, CDCl 3 ) δ 9.77 (s, 1H), 7.72 (d, J = 8.7 Hz, 2H), 6.92(d, J= 8.7 Hz, 2H), 4.10 (t, J = 5.7 Hz, 2H), 3.69 – 3.54 (m, 4H), 2.73 (t, J = 5.7 Hz, 2H), 2.54 – 2.40 (m, 4H).
[0042] (2) Preparation of Compound 2
[0043]
[0044] Dissolve compound 1 in tetrahydrofuran, add 2.2 equivalents of 2,4-dimethylpyrrole, add 0.2 mL of trifluoroacetic acid, react a...
Embodiment 2
[0055] The preparation steps of the probe are as follows:
[0056] (1) Preparation of compound 1
[0057] N 2 Under the protected ice-water bath, add 1 equivalent of triphenylphosphine into the tetrahydrofuran solvent to obtain a mixed solvent, dissolve 2-morpholine ethanol and p-hydroxybenzaldehyde in the mixed solvent according to the equivalent ratio of 1:1, and then add 1.5 An equivalent amount of diisopropyl azodicarboxylate was reacted at room temperature for 12 h, the solvent was removed under reduced pressure, and compound 1 was obtained by column chromatography;
[0058] (2) Preparation of compound 2
[0059] Dissolve compound 1 in tetrahydrofuran, add 2 equivalents of 2,4-dimethylpyrrole, add 0.1 mL trifluoroacetic acid, react at room temperature for 15 h, then add 1.5 equivalents of tetrachlorobenzoquinone, react at room temperature for 0.5 h, follow Add triethylamine and boron trifluoride diethyl ether in a ratio of 5:8 equivalent, react at room temperature for ...
Embodiment 3
[0065] The preparation steps of the probe are as follows:
[0066] (1) Preparation of compound 1
[0067] N 2 Under the protective ice-water bath, 1.5 equivalents of triphenylphosphine was added to the tetrahydrofuran solvent to obtain a mixed solvent, and 2-morpholine ethanol and p-hydroxybenzaldehyde were dissolved in the mixed solvent according to the equivalent ratio of 1:1.5, and then 1 An equivalent amount of diisopropyl azodicarboxylate was reacted at room temperature for 18 h, the solvent was removed under reduced pressure, and compound 1 was obtained by column chromatography;
[0068] (2) Preparation of compound 2
[0069] Dissolve compound 1 in tetrahydrofuran, add 3 equivalents of 2,4-dimethylpyrrole, add 0.2 mL of trifluoroacetic acid, react at room temperature for 12 h, then add 1.1 equivalent of tetrachlorobenzoquinone, react at room temperature for 0.8 h, follow Add triethylamine and boron trifluoride diethyl ether at a ratio of 7:9, react at room temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com