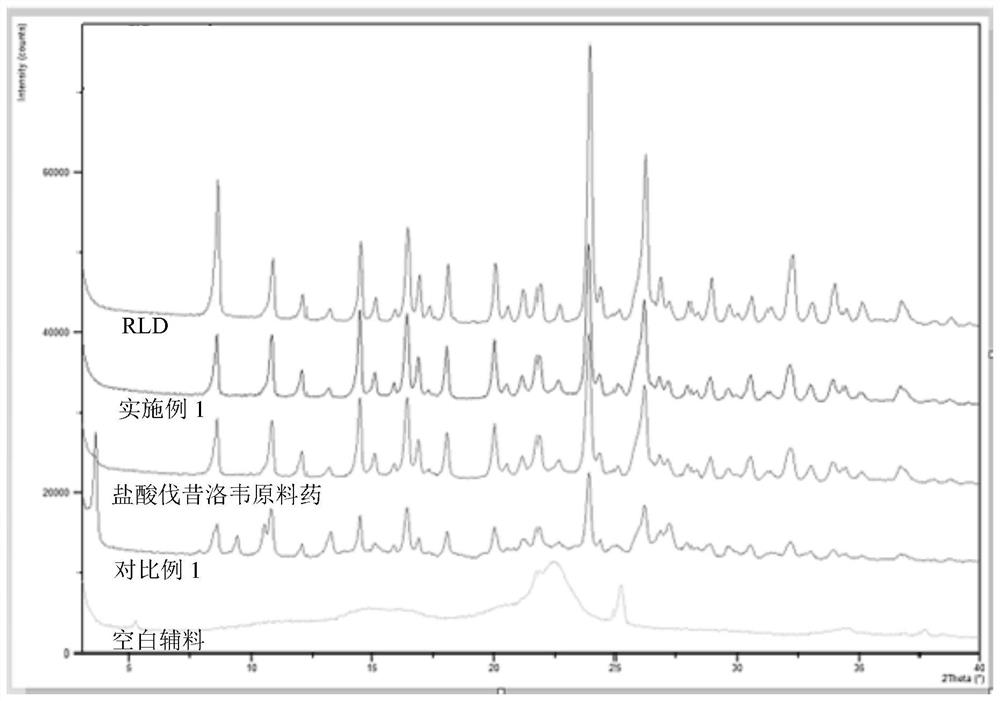
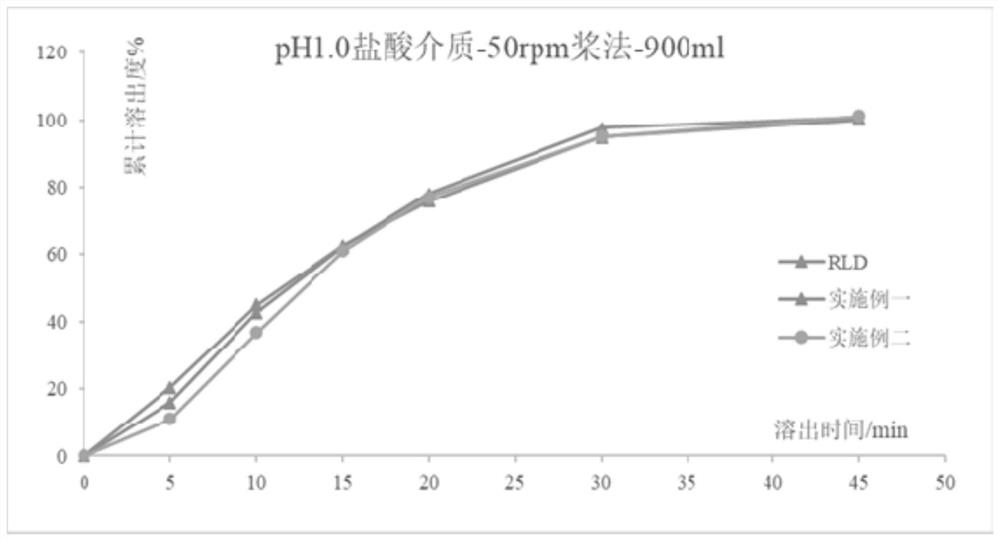
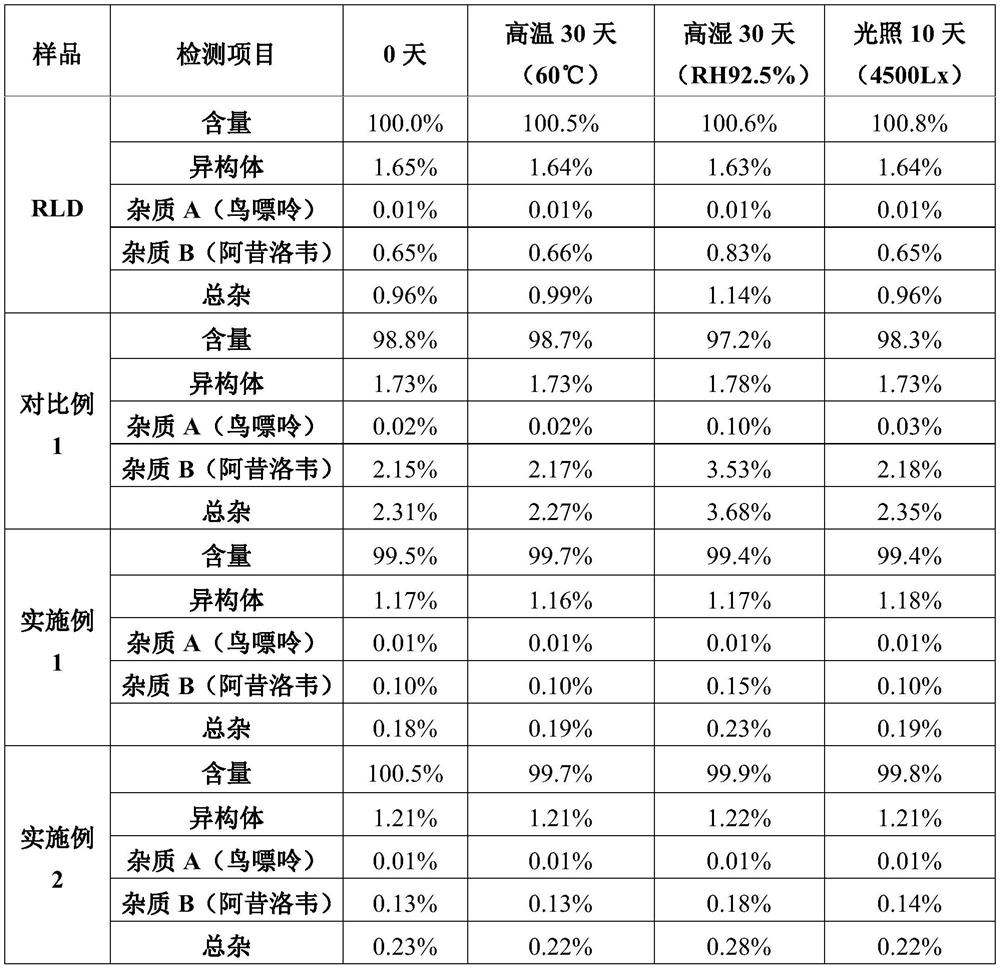
Valaciclovir hydrochloride tablet and preparation method thereof
A technology of valacyclovir hydrochloride and Lovir tablets, which is applied in the field of medicine and can solve the problems of many fine powders, complex procedures, and poor molding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The first aspect of the embodiment of the present application provides a method for preparing valacyclovir hydrochloride tablets, comprising the following steps:
[0029] S10. Adding valacyclovir hydrochloride, the first filler and the binder into the fluidized bed for fluidized bed granulation to obtain core granules;
[0030] S20. After mixing the inner core granules with the second filler, disintegrating agent and lubricant, the tablet is compressed to prepare the valacyclovir hydrochloride tablet core.
[0031] The preparation method of valacyclovir hydrochloride tablets provided in the first aspect of the application adopts the principle of fluidized bed granulation, utilizes high-speed hot air flow to suspend valacyclovir hydrochloride and fillers into a fluidized state, and passes through the adhesive The solution is sprayed and dried to form a liquid bridge, so that the fluidized material powder is combined into porous particles to obtain core particles. Then, ...
Embodiment 1
[0074] A kind of valacyclovir hydrochloride sheet, its preparation comprises steps:
[0075] Take by weighing valacyclovir hydrochloride 556g (D 90183 μm), microcrystalline cellulose 30g, added to the fluidized bed, weighed 24g of povidone, made into a solution, and carried out fluidized bed granulation with a fluidized bed, set the inlet air temperature: 60 ℃, material temperature 30 ℃, fan frequency 15-25Hz, start fluidization, set the peristaltic pump speed at 5-12rpm, spray slurry, after drying, the final moisture content is 2.65% MC, pass through a 24-mesh sieve for granulation. The ratio of obtained particles above 100 mesh to fine powder below 100 mesh is 67.2:32.8.
[0076] Take microcrystalline cellulose 30g, crospovidone 14g, colloidal silicon dioxide 4g, magnesium stearate 2g, add in the three-dimensional mixer together with granule, mix 10min, granule fluidity is good, carry out tabletting, pack coating, valacyclovir hydrochloride tablets can be obtained.
Embodiment 2
[0078] A kind of valacyclovir hydrochloride sheet, its preparation comprises steps:
[0079] Weigh valacyclovir hydrochloride 571g (D 90 258μm), 30g of lactose was added to the fluidized bed, 20g of cornstarch was weighed, and made into a starch slurry solution, and the fluidized bed was used for fluidized bed granulation. The fan frequency is 15-25Hz, fluidization is started, the speed of the peristaltic pump is set at 5-12rpm, and the slurry is sprayed. After drying, the final moisture content is 3.16% MC, and the granules are sieved through a 24-mesh sieve. The ratio of obtained particles above 100 mesh to fine powder below 100 mesh is 53.7:46.3.
[0080] Weigh 30g of lactose, 40g of dextrin, 26g of carboxymethyl cellulose, 4g of silicon dioxide, and 8g of sodium lauryl sulfate, and add them into a three-dimensional mixer together with the granules, and mix for 10 minutes. Valacyclovir hydrochloride tablets can be obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com