A porous ion-conducting membrane with gradient distribution of pore size and its preparation and application
An ion-conducting membrane and gradient distribution technology, used in fuel cells, regenerative fuel cells, electrical components, etc., can solve the problems of reducing membrane ion selectivity and ion conductivity, and achieve improved selectivity and ion conductivity. Effect of battery performance, fast ion conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
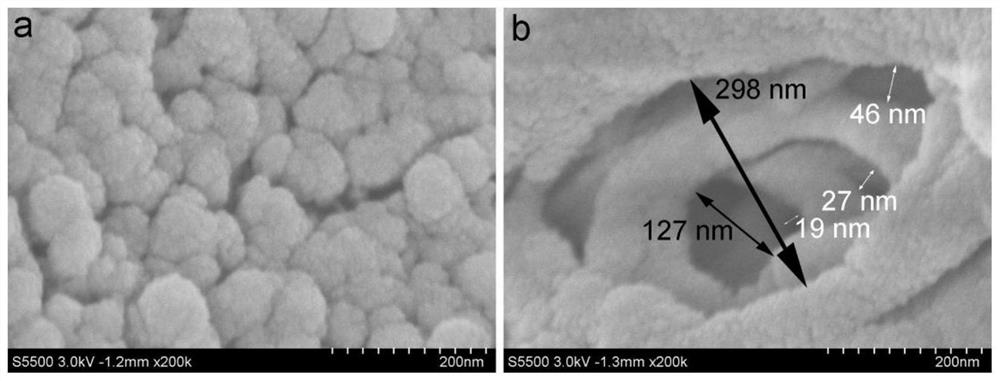
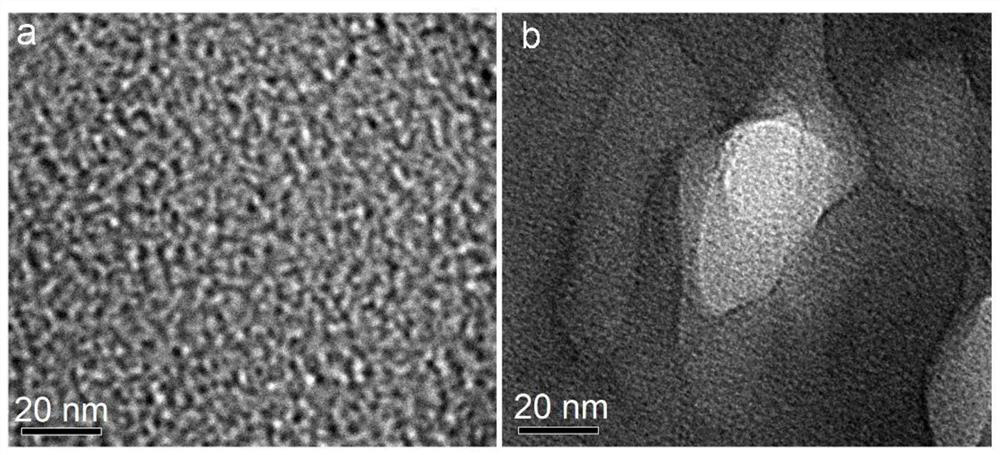
[0043] Using PES / PVP as the base material, dissolve PES / PVP in DMAC solvent to obtain a blend solution with a mass concentration of 35%, wherein the mass ratio of PES to PVP is 85:15. After the solution is completely dissolved, add 1.2g of carbonic acid Sodium hydrogen (accounting for 43% of the polymer resin, particle size 5-15nm), and uniformly stirred and dispersed; the above-mentioned blended solution was poured on a clean and flat glass plate, and the solvent was volatilized for 10s under 20% humidity, and then the The whole is immersed in a 10wt% hydrochloric acid solution for 3 minutes, and prepared at 25° C. to form a porous ion-conducting membrane, referred to as a P-P membrane. Characterize its microscopic morphology. From figure 1 From the SEM image of b, it can be seen that the cross-sectional cortex structure of the prepared P-P membrane has an obvious pore structure; at the same time, the pore size has an obvious gradient distribution, and the distribution chang...
Embodiment 2
[0046] Using PES / PVP as the base material, dissolve PES / PVP in DMAC solvent to obtain a blend solution with a mass concentration of 35%, wherein the mass ratio of PES to PVP is 85:15. After the solution is completely dissolved, add 1.6g of carbonic acid sodium hydrogen (accounting for 57% of the polymer resin), and uniformly stir and disperse; pour the above blended solution on a clean and flat glass plate, evaporate the solvent for 10s under 20% humidity, and then immerse it as a whole in 10wt% hydrochloric acid In the solution, a porous ion-conducting membrane with a gradient distribution of pore diameters is prepared at 25°C. The size of the large pores is 2.8 μm to 3.2 μm; the size of the middle pores is 235 nm to 260 nm; the size of the small pores is 0.9 to 1.4 nm. 500nm, the thickness of the macroporous support layer is about 100um, and the porosity of the membrane is 80% to 85%. Due to the increase in the content of sodium bicarbonate added, more bubbles are generated ...
Embodiment 3
[0048] Using PES / PVP as the base material, dissolve PES / PVP in DMAC solvent to obtain a blend solution with a mass concentration of 35%, wherein the mass ratio of PES to PVP is 85:15. After the solution is completely dissolved, add 1.2g of carbonic acid Sodium (accounting for 43% of the polymer resin), and uniformly stirred to disperse; pour the above blended solution on a clean and flat glass plate, evaporate the solvent for 10s under 20% humidity, and then immerse it as a whole in 10wt% hydrochloric acid solution In 25°C, a porous ion-conducting membrane with a gradient distribution of pore sizes was prepared. The macropore size is 3.0μm~3.2μm; the mesopore size is 240nm~260nm; the small pore size is 1.0~1.4nm, the thickness of the cortex is about 500nm, the thickness of the macropore support layer is about 100um, and the porosity of the membrane is 70%~ 85%. Because sodium carbonate is added, in the film forming process, the hydrochloric acid in the coagulation bath first ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com