Application of ligustrazine nitrone compound in the preparation of prevention and/or treatment of muscular dystrophy diseases
A technology of ligustrazine nitrone and compounds, which is applied in the field of application of ligustrazine nitrone compounds in the prevention and treatment of muscular dystrophy diseases, can solve problems such as exceeding the threshold of cost-benefit ratio, delay the process of disease deterioration, and increase profits /hazard ratio, efficacy-enhancing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Construction and grouping of embodiment 1.DMD model mice
[0059] (1) Establishment of animal model of Duchenne muscular dystrophy
[0060] Entrusted Nanjing University-Nanjing Institute of Biomedicine to construct the gRNA of the mouse Dmd gene, using CRISPR / Cas9 technology and blastocyst injection technology to target Exon4 of the mouse Dmd gene, and screen for mouse models that can cause frameshift mutations in the Dmd gene.
[0061] (2) Animal grouping and administration
[0062] The mice were kept in the SPF animal laboratory of the Experimental Animal Center of Sanyuanli Campus, Guangzhou University of Traditional Chinese Medicine. Free access to water and food.
[0063]
[0064] Administration method: The animals in each group were administered by intragastric administration for 24 weeks from the age of 8 weeks, and different doses of TBN were intragastrically administered twice a day; the positive control drug deflazacort was administered intraperitoneally,...
Embodiment 2
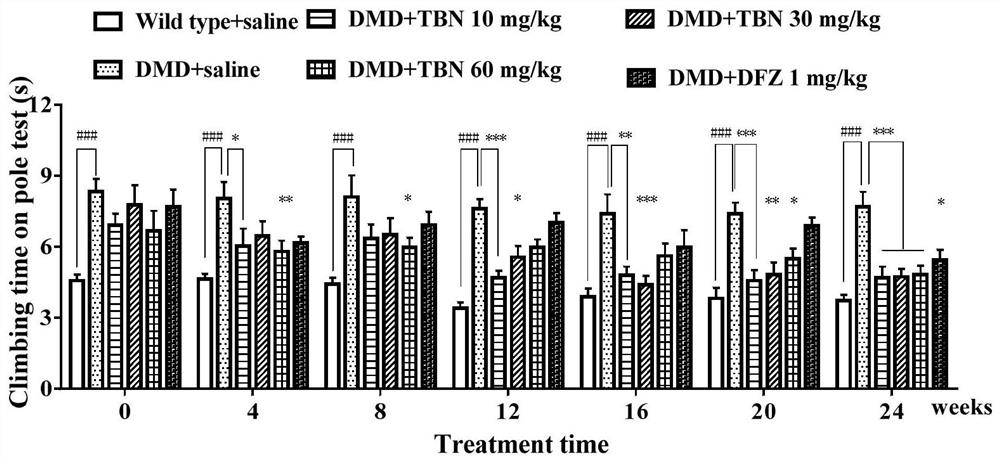
[0065] Example 2. Effects of TBN on motor coordination ability and motor ability of DMD mice.
[0066] In order to evaluate the improvement of TBN on the motor behavior function of DMD mice, after the administration started, the pole climbing time and open field movement distance of the mice in each group were measured every 4 weeks for 24 consecutive weeks.
[0067] The pole-climbing test can assess the movement and coordination of the mouse limbs. Make a wooden pole with a length of about 50cm and a diameter of about 1cm. The pole is wrapped with gauze to increase friction. The wooden pole is placed vertically on a horizontal table, and the mouse is gently placed on the top of the pole with its head down. The mouse crawls down autonomously without being driven by external force, and the time for the mouse to climb from the top of the pole to the platform at the bottom is recorded. (rod climbing time). Continuous training for 3 days before each measurement, 2 times a day, the...
Embodiment 3
[0069] Embodiment 3.TBN significantly improves the content of SOD in DMD mouse serum
[0070] At the end of the experiment, blood was taken from the abdominal aorta of the anesthetized mice, and after standing for 1 hour, the supernatant was collected by centrifugation at 3000rmp for 10 minutes, and the content was measured by an automatic biochemical analyzer according to the instructions of the SOD kit. Research results such as image 3 As shown, compared with the DMD model group, the 30mg / kg TBN group significantly increased the content of SOD in the serum, and the therapeutic effect was better than that of the positive drug deflazacort.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com