Anti-NGF antibody and antigen binding fragment thereof, and preparation method and application thereof
A technology for binding fragments and antibodies, which is applied in the preparation of the antibodies and their antigen-binding fragments, and in the field of anti-NGF antibodies and their antigen-binding fragments, which can solve the problems of different immunogenicity, different antibody tolerance speed and toxicity, and influence on drug efficacy And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] Embodiment 1: the preparation of murine source anti-NGF antibody
[0149] Recombinant human NGF protein (Beijing Yiqiao Shenzhou Technology Co., Ltd., 11050-HNAC) was mixed and emulsified with an equal amount of complete Freund's adjuvant (Sigma-Alderich, F5881) as an immunogen for initial immunization. Five 6-week-old BALB / c mice and five C57 mice (Jiangsu Huafukang) were prepared, and each animal was subcutaneously injected with 50 μg of immunogen (excluding adjuvant quality, the same below). The immunogen was mixed and emulsified with Freund's incomplete adjuvant (Sigma-Alderich, F5506) for subsequent booster immunization. Two weeks after the initial immunization, each animal was intraperitoneally injected with 25 μg of immunogen for the first booster; two weeks later, each animal was subcutaneously injected with 25 μg of immunogen for the second booster. 4-5 weeks after the last immune shock, 25 μg of immunogen was injected intraperitoneally.
[0150] After the ...
Embodiment 2
[0152] Example 2: ELISA detection of murine anti-NGF antibody binding to human NGF
[0153] The binding ability of the anti-NGF antibody was studied using human NGF (Beijing Sino Biological Science and Technology Co., Ltd., 11050-HNAC) as the antigen. Each well of the 96-well ELISA plate was coated with 50ng human NGF, after washing and blocking, the antibody was added in gradient dilution and incubated at room temperature for 1 hour. After washing three times, add HRP-conjugated goat anti-mouse antibody (Biolegend, 405306), incubate at room temperature for 1 hour, add tetramethylbenzidine (TMB, Biolegend, 421101) to develop color after washing three times, stop color development with 1M HCl, and enzyme label The instrument reads the absorbance at 450nm.
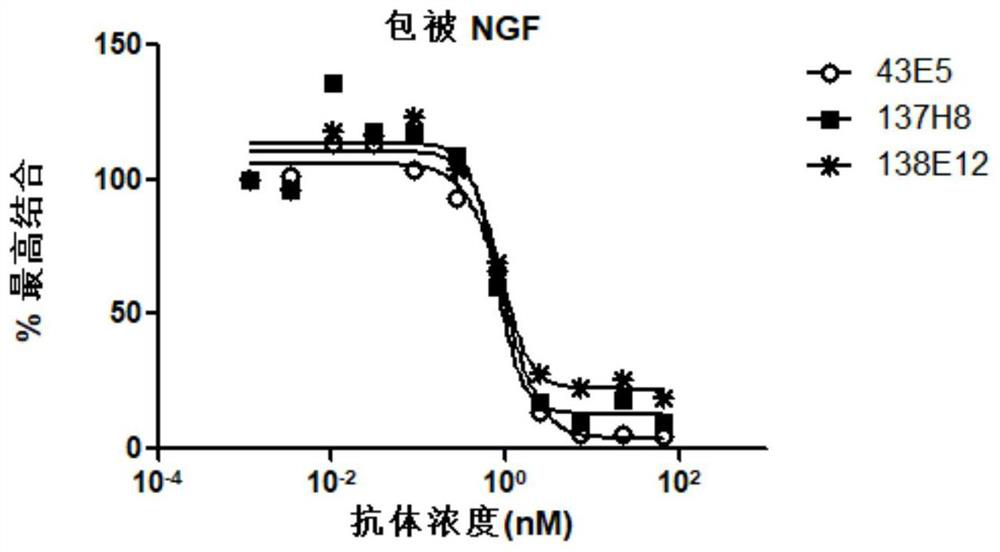
[0154] Anti-NGF antibodies secreted by the three hybridomas all bound to human NGF in a dose-dependent manner ( figure 1 ), the EC50s of the three antibodies binding to human NGF were 0.082nM, 0.112nM, and 0.1nM, respect...
Embodiment 3
[0157] Example 3: Detection of binding affinity between murine anti-NGF antibody and human NGF
[0158] Antibody affinity was determined using the biomolecular interaction detection platform ForteBio Octet Red96 (PALL). Biotin-labeled human NGF was immobilized using SA (Streptavidin) Biosensor (Fortebio, 18-5021), and then combined with gradient concentrations of anti-NGF antibodies. Buffer (1X Kinetics Buffer: PBS+0.1%BSA+0.05%Tween20) was added for dissociation, and finally the affinity kinetic constant of antigen-antibody binding was calculated by instrument algorithm (Table 2).
[0159] Table 2 Binding affinity of anti-NGF antibodies to human NGF
[0160]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com