A kind of composite crystal, preparation method and application
A compound and crystal technology, applied in the field of chemical medicine, can solve the problem of not providing crystal structure data, etc., and achieve the effects of prolonging the onset time, delaying the dissolution rate, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
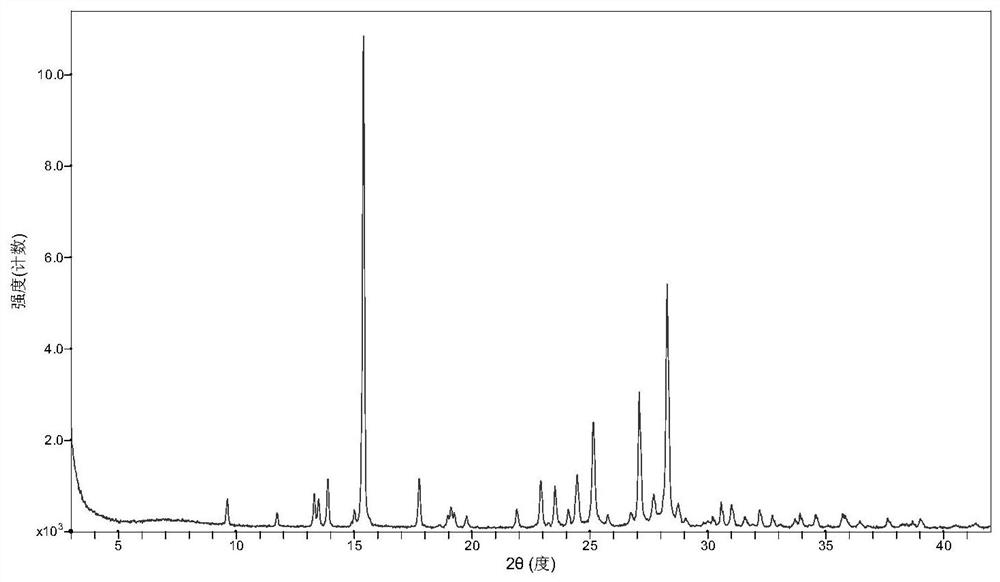
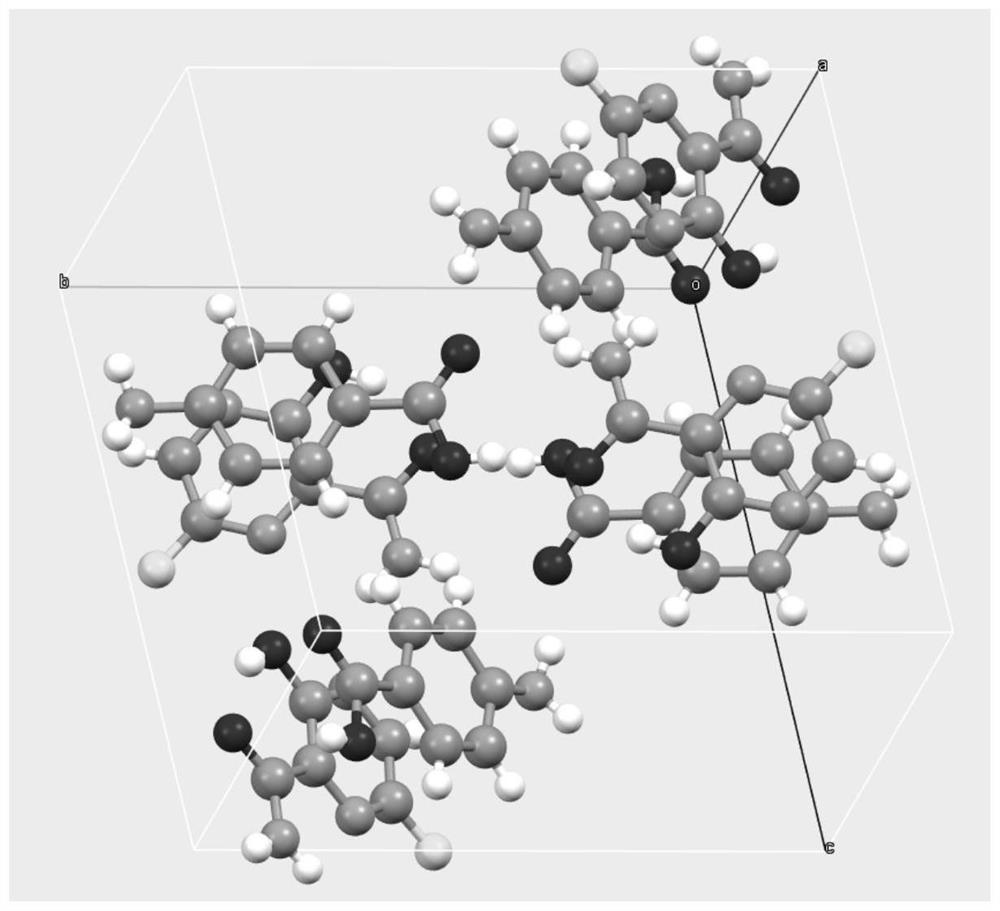
[0031] After mixing favipiravir with p-aminobenzoic acid in a molar ratio of 1:1, take about 2g of solids, add about 0.5 ml of ethyl acetate and manually grind in a mortar for 30 min. Samples are placed in a blast drying chamber and dried at 30 °C. The resulting product was XRD analyzed as a eutectic of favipiravir-p-aminobenzoic acid.
Embodiment 2
[0033] After mixing favipiravir and p-aminobenzoic acid in a molar ratio of 1:1, about 2g of solids were taken, 10ml of methyl tert-butyl ether was added, and magnetic stirring was stirred at room temperature for 72h to 96h. After the sample is filtered, the resulting solids are placed in a blast drying box and dried at 60 °C. The resulting solids were analyzed by XRD and showed that they were eutectic of favipiravir-p-aminobenzoic acid.
Embodiment 3
[0035] Take 0.6 g of p-aminobenzoic acid, add 50 ml of ethyl acetate, heat and dissolve. Add 0.456 g of favipiravir and stir for 2h. The solids were filtered and dried in a blast at 40 °C, and XRD analysis showed that they were eutectic of fapilabvir-p-aminobenzoic acid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com