Preparation method of lipoic acid
A technology of lipoic acid and lipoic acid ethyl ester, applied in directions such as organic chemistry, can solve the problems of affecting the yield and purity of lipoic acid, material waste, complicated reaction process, etc., and achieves the cost saving of raw materials, shortening reaction time and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
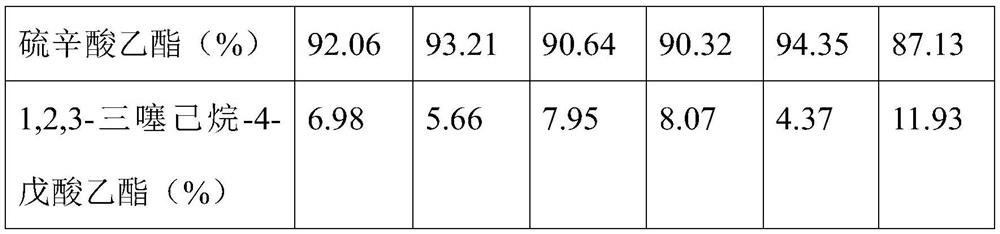
Embodiment 1
[0023] (1) Take sodium sulfide in a beaker, add distilled water to the beaker, heat and stir it below 50°C to dissolve it, and make a 3mol / L sodium sulfide solution; add sulfur to the obtained sodium sulfide solution, stir to dissolve it, Make the concentration of sulfur in the sodium sulfide solution be 3.5mol / L to obtain a mixed solution;
[0024] (2) Add 6,8-dichlorooctanoic acid ethyl ester and distilled water successively in photoreactor and be mixed with 3mol / L 6,8-dichlorooctanoic acid ethyl ester solution, then add phase transfer catalyst in solution, stir and heat up to 82-84°C;
[0025] (3) Add the mixed solution obtained in step (1) dropwise to the reaction solution in step (2) within 180min at a volume ratio of 5:3.5, while using a 1000W xenon lamp and using a 680nm cut-off filter to process the reaction solution. Photocatalysis, and stirring the reaction solution at a speed of 200r / min;
[0026] (4) Add sodium sulfite to the photoreactor after the cyclization re...
Embodiment 2
[0029] (1) Take sodium sulfide in a beaker, add distilled water to the beaker, heat and stir it below 50°C to dissolve it, and make a 3mol / L sodium sulfide solution; add sulfur to the obtained sodium sulfide solution, stir to dissolve it, Make the concentration of sulfur in the sodium sulfide solution be 3.5mol / L to obtain a mixed solution;
[0030] (2) Add 6,8-dichlorooctanoic acid ethyl ester and distilled water successively in photoreactor and be mixed with 3mol / L 6,8-dichlorooctanoic acid ethyl ester solution, then add phase transfer catalyst in solution, stir and heat up to 82-84°C;
[0031] (3) Add the mixed solution obtained in step (1) dropwise to the reaction solution in step (2) at a volume ratio of 5:3 within 180 minutes, while using a 1000W xenon lamp and using a 680nm cut-off filter to filter the reaction solution. Photocatalysis, and stirring the reaction solution at a speed of 200r / min;
[0032] (4) Add sodium sulfite to the photoreactor after the cyclization ...
Embodiment 3
[0035] (1) Take sodium sulfide in a beaker, add distilled water to the beaker, heat and stir it below 50°C to dissolve it, and make a 3mol / L sodium sulfide solution; add sulfur to the obtained sodium sulfide solution, stir to dissolve it, Make the concentration of sulfur in the sodium sulfide solution be 3.5mol / L to obtain a mixed solution;
[0036] (2) Add 6,8-dichlorooctanoic acid ethyl ester and distilled water successively in photoreactor and be mixed with 3mol / L 6,8-dichlorooctanoic acid ethyl ester solution, then add phase transfer catalyst in solution, stir and heat up to 82-84°C;
[0037] (3) Add the mixed solution obtained in step (1) dropwise to the reaction solution in step (2) at a volume ratio of 5:4 within 180 minutes, while using a 1000W xenon lamp and using a 680nm cut-off filter to filter the reaction solution. Photocatalysis, and stirring the reaction solution at a speed of 200r / min;
[0038] (4) Add sodium sulfite to the photoreactor after the cyclization ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com