Sustained-release pharmaceutical compositions comprising an antipsychotic drug and uses thereof
A technology of antipsychotic drugs and compositions, which is applied in the direction of drug combinations, medical preparations containing active ingredients, medical preparations with non-active ingredients, etc., and can solve the problem of reducing the frequency of antipsychotic drug administration and slow-release drugs with high characteristics Embedding rate and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0048] Example 1, the preparation of aripiprazole liposome formulation
[0049]Empty liposomes were prepared by the lipid film hydration-extrusion method. HSPC, cholesterol and DSPE-PEG2000 (59.5 / 39.6 / 0.9 mole percent) were dissolved in chloroform, and the organic solvent was removed under vacuum by a rotary evaporator to form a thin lipid film. The dried lipid film was hydrated with 75 mM triethylammonium sucrose octasulfate (pH 6.0) at 60° C. for 30 minutes to form liposomes with aqueous core entrapped with triethylammonium sucrose octasulfate. After six freeze-thaw cycles between liquid nitrogen and 60°C water, liposomes were squeezed 10 times through a polycarbonate filter with a pore size of 0.2 μm. Unentrapped triethylammonium sucrose octasulfate was removed by dialysis against a 9.4% sucrose solution.
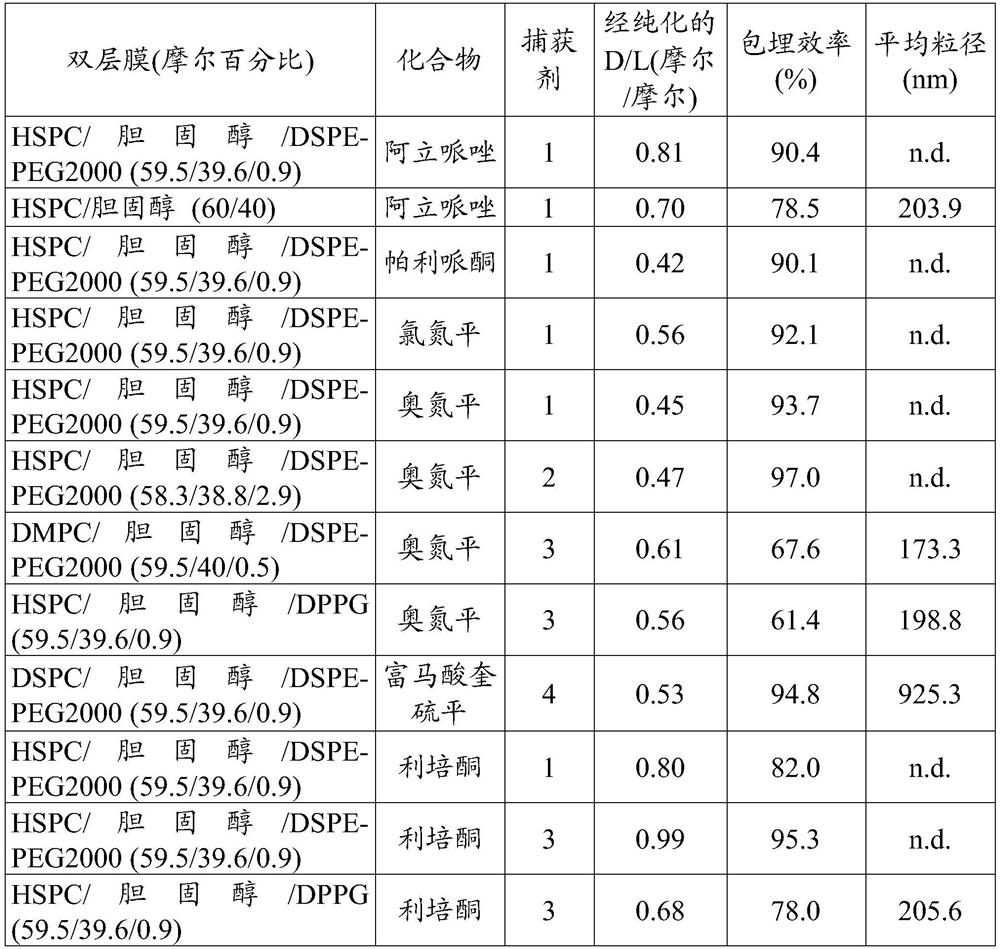
[0050] Containing 1.0 mg / mL aripiprazole (Abblis Chemicals LLC, USA), empty liposomes (containing 2.5 mM lipid) prepared according to the previous paragraph, 100 mM ci...
example 2
[0053] Example 2, the preparation of olanzapine liposome formulation
[0054] Empty liposomes were prepared according to Example 1. The bilayer membrane contains HSPC, cholesterol and DPPG (59.5 / 39.6 / 0.9 mole percent) and the capture agent is 300 mM ammonium sulfate.
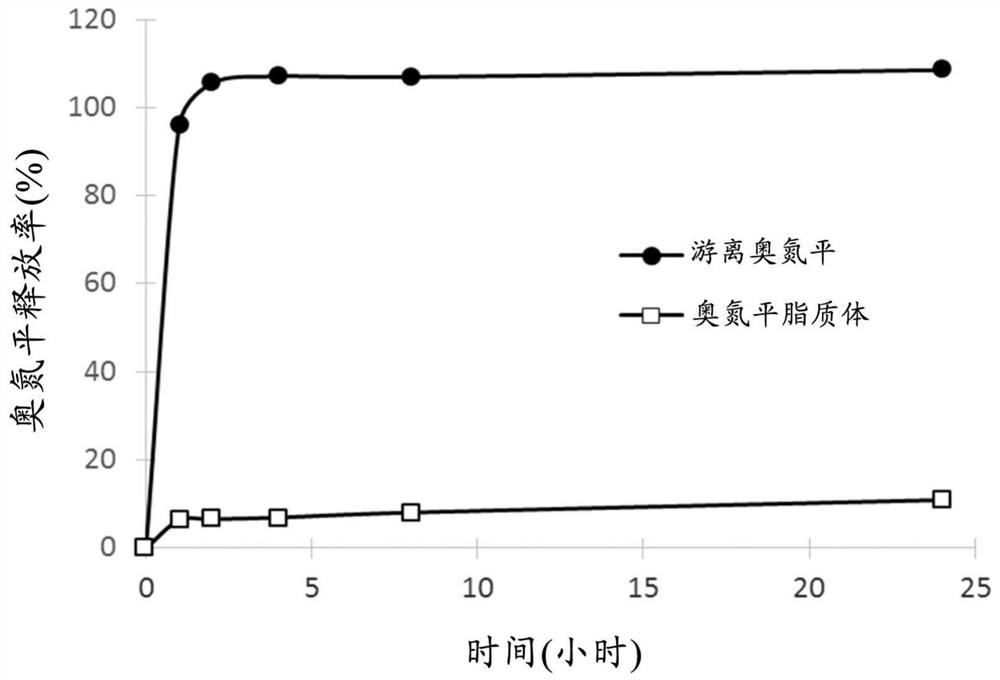
[0055] A reaction mixture containing 6.1 mg / mL olanzapine (TCI America, US), empty liposomes of 21.3 mM lipid, and 35 mM histidine buffer (pH 7.35) was incubated at 60° C. for 15 minutes, and then passed through Sephadex TM Unentrapped olanzapine was isolated with G-50 fine gel (GE Healthcare) or dialysis bags (Spectrum Labs) in 9.4% sucrose solution. The olanzapine (drug) concentration and lipid concentration of the reaction mixture and olanzapine-embedded liposomes were measured using an ultraviolet / visible (UV / Vis) spectrometer. D / L and entrapment efficiency of olanzapine liposome formulation are calculated according to example 1. The particle size distribution was measured by a dynamic light scattering i...
example 3
[0057] Example 3, the preparation of risperidone liposome formulation
[0058] Empty liposomes were prepared according to Example 1. The bilayer membrane contains HSPC, cholesterol and DSPE-PEG2000 (59.5 / 39.6 / 0.9 mole percent) and the capture agent is 300 mM ammonium sulfate.
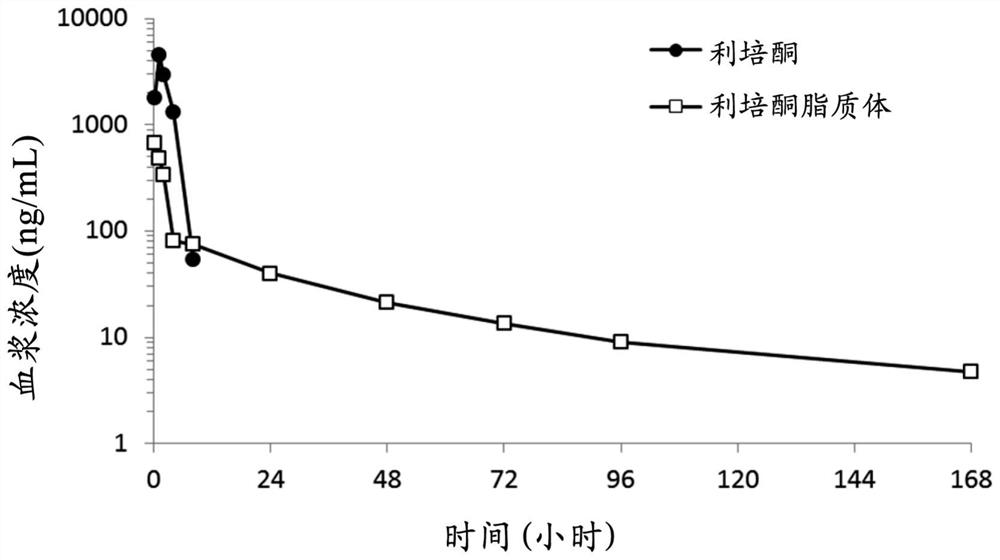
[0059] A reaction mixture containing 5.4 mg / mL risperidone (TCI America, US), empty liposomes of 24.4 mM lipid, and 20 mM histidine buffer (pH 6.5) was incubated at 60° C. for 15 minutes, and then passed through Sephadex TM Unentrapped risperidone was isolated with G-50 fine gel (GE Healthcare) or dialysis bags (Spectrum Labs) in 9.4% sucrose solution. The risperidone (drug) concentration and lipid concentration of the reaction mixture and risperidone-embedded liposomes were measured using an ultraviolet / visible (UV / Vis) spectrometer. The D / L and embedding efficiency of risperidone liposome formulation are calculated according to example 1.
[0060] Using 300 mM ammonium sulfate as capture agent, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com