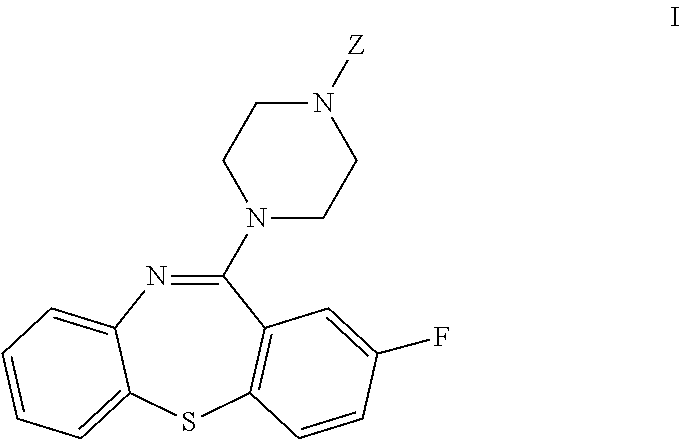
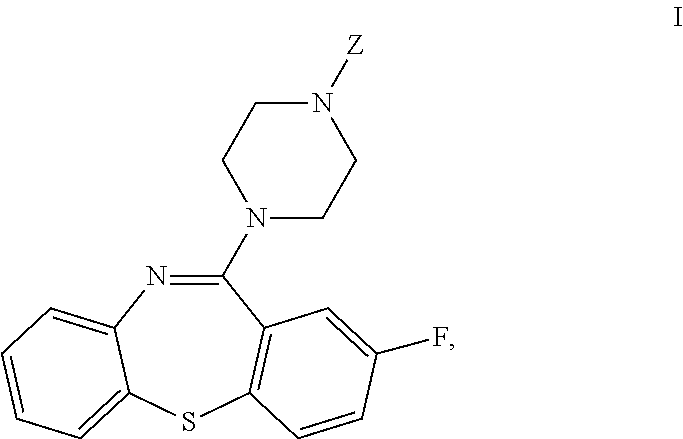
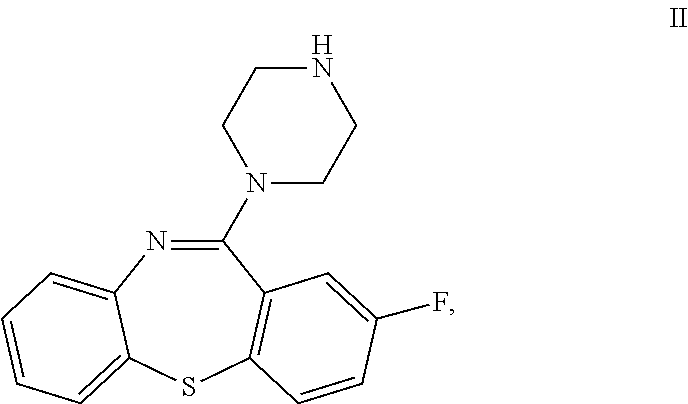
Dibenzothiazepine Derivatives and Use Thereof
a technology of dibenzothiazepine and derivatives, applied in the field of bipolar disorders, mood disorders, anxiety disorders, etc., can solve the problems of high treatment compliance, low remission rate, safety and tolerability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1a
2-fluoro-11-(piperazin-1-yl)dibenzo[b,f][1,4]thiazepine
[0101]Scheme A shows one method of preparing 2-fluoro-11-(piperazin-1-yl)dibenzo[b,f][1,4]thiazepine (VI).
[0102]Thus, in a five-step process: 5-fluoro-2-mercapto-benzoic acid ethyl ester may be reacted with 1-fluoro-2-nitrobenzene to form 5-fluoro-2-(2-nitro-phenylsulfanyl)-benzoic acid ethyl ester; the ethyl ester may be converted to 5-fluoro-2-(2-amino-phenylsulfanyl)-benzoic acid ethyl ester; the aminophenyl compound may be cyclized to form 2-fluoro-10H-dibenzo[b,f][1,4]thiazepin-11-one, that may be converted to 11-chloro-2-fluoro-dibenzo[b,f][1,4]thiazepine, and that may be then reacted with piperazine to form the title compound.
Step 1:
[0103]To a solution of ethyl 5-fluoro-2-mercaptobenzoate (I) (25.0 g, 124.9 mmol) and 1-fluoro-2-nitrobenzene (13.2 mL, 124.9 mmol) in acetone (700 mL) was added K2CO3 (34.5 g, 249.7 mmol) at ambient temperature. The yellow suspension was heated to reflux (60° C.) for 5 hours. The reaction mix...
example 1b
2-fluoro-11-(piperazin-1-yl)dibenzo[b,f][1,4]thiazepine
[0108]Scheme B shows another contemplated method of preparing 2-fluoro-11-(piperazin-1-yl)dibenzo[b,f][1,4]thiazepine.
[0109]Thus, in a three-step process: 2,2′-disulfanediyldianiline may be reacted with 2-bromo-5-fluoro-benzoic acid, as shown, to form 2-(2-amino-phenylsulfanyl)-5-fluoro-benzoic acid; the benzoic acid may be cyclized, as shown, to form 2-fluoro-10H-dibenzo[b,f][1,4]thiazepin-11-one, that may be converted to 11-chloro-2-fluoro-dibenzo[b,f][1,4]thiazepine, and that may be reacted with piperazine to form the title compound.
Synthesis of 2-fluorodibenzo[b,f][1,4]thiazepin-11(10H)-one
[0110]
[0111]A suspension of nickel bromide (5 mmol, 1.09 g), bipyridyl (5 mmol, 0.78 g) and zinc dust (200 mmol, 13.08 g) in 200 mL dry acetonitrile was magnetically stirred, treated with 2,2′-disulfanediyldianiline (52 mmol, 12.92 g) and heated in an oil bath set at 75° C. for 30 min after reaching maximum internal temperature. At the end...
example 1c
2-fluoro-11-(piperazin-1-yl)dibenzo[b,f][1,4]thiazepine
[0114]Scheme C shows another contemplated method of preparing 2-fluoro-11-(piperazin-1-yl)dibenzo[b,f][1,4]thiazepine.
[0115]Thus, 1-chloro-2-nitrobenzene may be reacted with 4-fluorobenzenethiol in the presence of a base to form 1-nitro-2-phenylsulfanyl-(4-fluorobenzene). The nitrofluorobenzene may be reduced to 1-amino-2-phenylsulfanyl-(4-fluorobenzene) which can converted (for example, as shown) to [2-(4-fluoro-phenylsulfanyl)-phenyl]-carbamic acid phenyl ester. Such an ester may be cyclized as shown to form 2-fluoro-10H-dibenzo[b,f][1,4]thiazepin-11-one, that may be converted to 11-chloro-2-fluoro-dibenzo[b,f][1,4]thiazepine, which can then be reacted with piperazine to form the title compound.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com