Self-microemulsion composition of tyrosine kinase inhibitor
A technology of tyrosine kinase and inhibitor, which is applied in the field of self-microemulsion composition of tyrosine kinase inhibitor, and can solve problems such as adverse reactions of the digestive system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
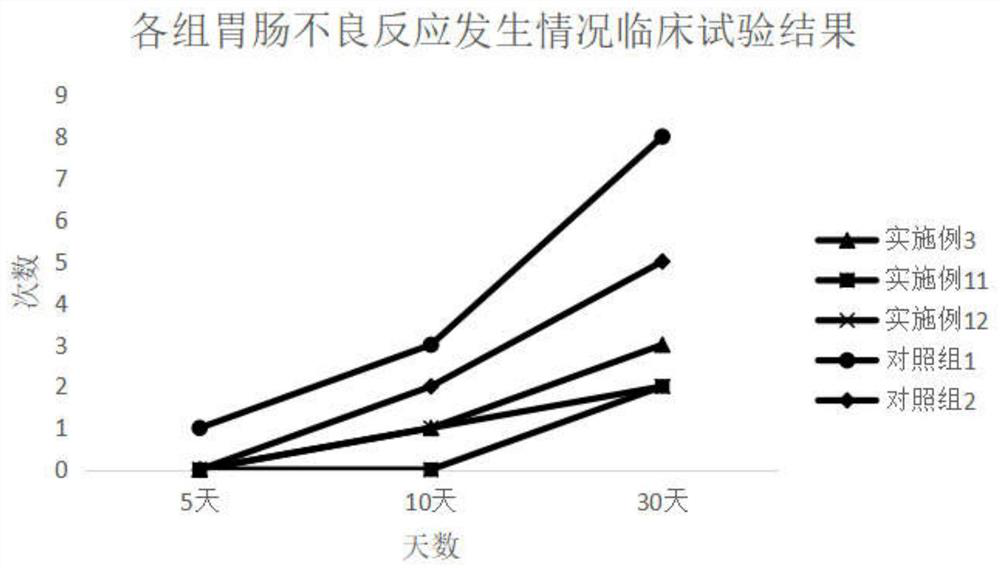
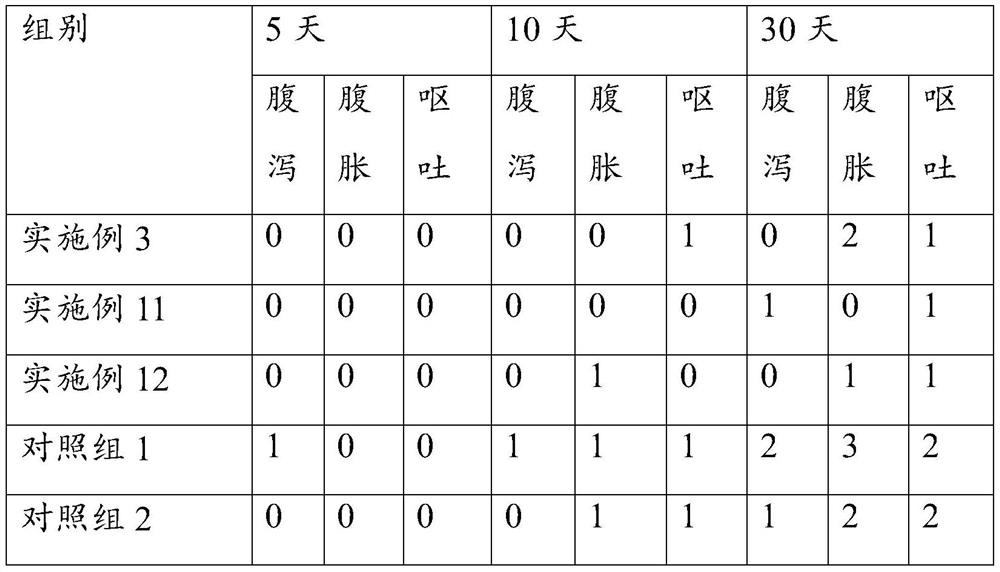
Embodiment 1
[0062] The prescription is as follows: Axitinib: 5mg;
[0063] The total mass of the SMEDDS carrier is 600mg: oil phase: surfactant: the mass ratio of co-surfactant is: 16.7:53.3:30; the oil phase is ethyl oleate; the surfactant is polyoxyethylene castor oil ; The co-surfactant is diethylene glycol monoethyl ether. The preparation process refers to Experiment 1, and the particle size measurement refers to Experiment 2. The particle diameter of the nanoemulsion of the obtained axitinib preparation is 18.45nm.
Embodiment 2
[0065] The prescription is as follows: lurasidone hydrochloride: 5mg;
[0066] The total mass of SMEDDS carrier is 400mg: (capryol90: M 1944CS):RH40:(PEG400:TP)=(13.3:6.7):53.3:(17.8:8.9), the preparation process refers to Experiment 1, and the particle size test refers to Experiment 2. The particle size of the obtained nanoemulsion is 26.24nm.
Embodiment 3
[0068] The prescription is as follows: lurasidone hydrochloride: 5mg;
[0069] The total mass of SMEDDS carrier is 600mg: (propylene glycol caprylate: oleic acid macrogol glyceride): polyoxyethylene hydrogenated castor oil: (polyethylene glycol 400: diethylene glycol monoethyl ether)=(13.3: 6.7):53.3:(17.8:8.9). The preparation process refers to Experiment 1, and the particle size measurement refers to Experiment 2. The particle size of the obtained nanoemulsion is 25.93nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com