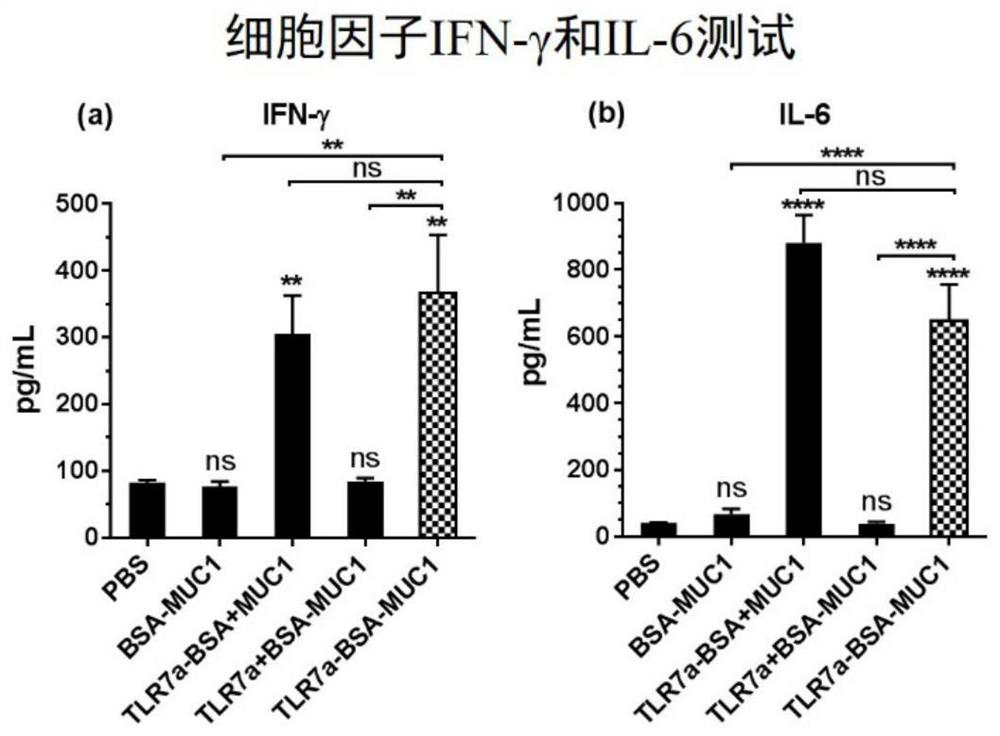
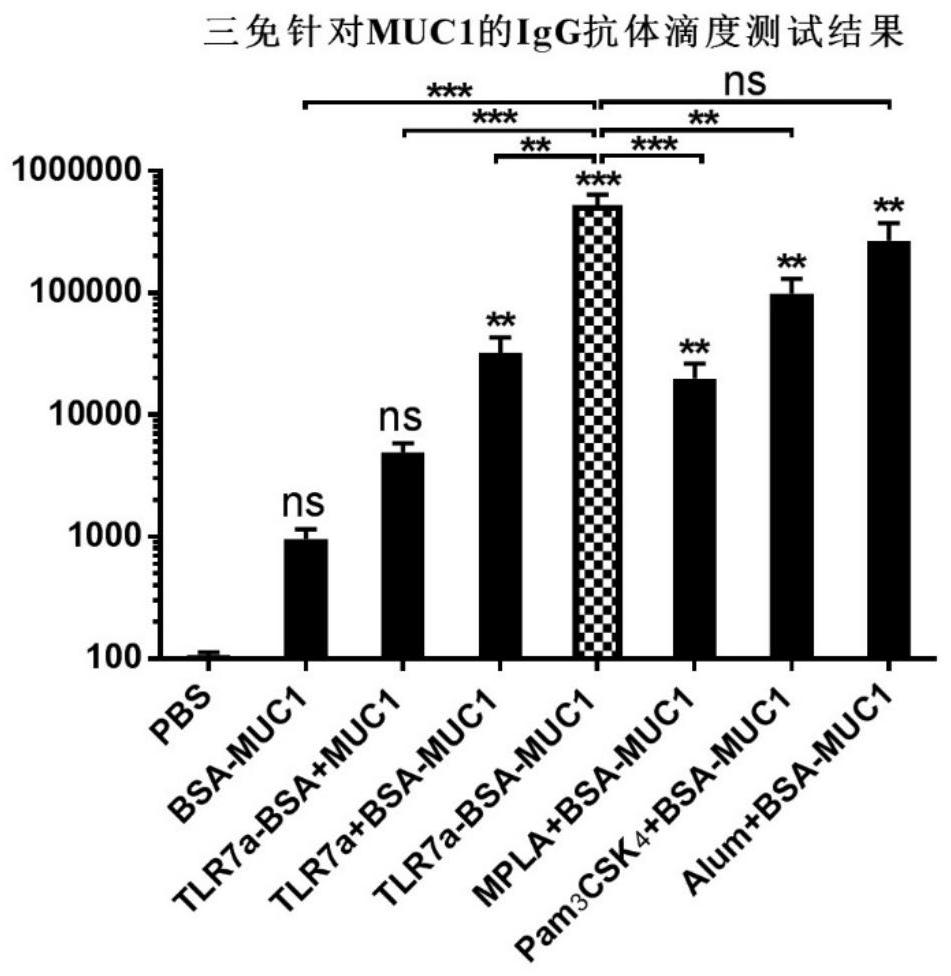
Anti-tumour vaccine molecule, and preparation method and application thereof
An anti-tumor and vaccine technology, applied in the field of anti-tumor vaccines, can solve the problems of poor immunogenicity of tumor-associated antigens, and achieve strong cellular immunity, good immune performance, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] Example 1: Preparation of TLR7 agonists
[0131]
[0132] Synthesis of compound 2: 2-chloroadenine (5.4g, 31.8mmol) and sodium (5.0g, 217mmol) were mixed in ethylene glycol monomethyl ether (235mL, 3.1mol), and the mixture was stirred and refluxed at 140°C for 10h . Then add 25 mL of water, add 1 mol / L hydrochloric acid until the pH value = 7, concentrate, wash with water and filter with suction to obtain compound 2, which is directly carried out to the next step without purification.
[0133] Synthesis of compound 3: Compound 2 (0.7g, 3.34mmol), potassium carbonate (3.2g, 23.44mmol), methyl 4-bromomethylbenzoate (1.5g, 6.68mmol) were added to 10mL of dry N,N-di Methylformamide (DMF), stirred and refluxed at 60°C for 8h. Then add a 5% aqueous solution of citric acid until no bubbles are generated, extract with chloroform, wash with brine, and MgSO 4 After drying, filtration, concentration, the reaction mixture was purified by column chromatography to give 3 as a w...
Embodiment 2
[0136] Example 2: Preparation of TLR7 agonist coupled with carrier protein BSA
[0137]
[0138] Synthesis of compound 14: under argon protection, compound 5 (0.03mmol) and EDCI (0.09mmol) were dissolved in DMF (1mL), and finally NHS (N-hydroxysuccinimide) (0.09mmol) was added, 25 Stirring at °C for 3 h; then the resulting mixture was spin-dried with an oil pump to obtain compound 13.
[0139] BSA (0.3μmol, purchased from Wuhan Chucheng Zhengmao Science and Technology Engineering Co., Ltd., brand name CC1050003) was dissolved in PBS (2mL), compound 13 (0.006mmol) was added to DMF and dissolved, the two solutions were mixed, and placed on a shaker at 25°C Reaction 48h. The obtained compound 14 (defined as TLR7a-BSA) was purified by centrifugal filtration with an ultrafiltration tube (Millipore UFC910096 15M, 10KD) and lyophilized. Tested by MALDI-TOF-MS, the average number of covalent linkages between TLR7a and BSA was calculated to be 6 to 7.
Embodiment 3
[0140] Example 3: Preparation of antigen MUC1
[0141]
[0142]
[0143] Compound 8 was synthesized by manual solid phase, and the operation steps were as follows:
[0144] (1) Activation of Rink Amide AM (purchased from Jill Biochemical (Shanghai) Co., Ltd.) resin
[0145] Weigh 306 mg (0.2 mmol) of Rink Amide AM resin into a solid-phase synthesis reaction tube, and use dry dichloromethane (DCM) (4 mL) to swell for 30 min under nitrogen protection. Wash: use dry DCM (3 x 3 mL) and dry ( Spherical molecular sieve dried) N,N-dimethylformamide (DMF) (3 × 3mL) alternately washed repeatedly.
[0146] (2) Fmoc group deprotection
[0147] Under nitrogen agitation, 20% piperidine / DMF solution (3 mL, 3×5 min) was added to the reaction tube, and washing: the resin was washed alternately and repeatedly with DCM (3×3 mL) and DMF (3×3 mL).
[0148] (3) Kaiser-test
[0149] Kaiser-test judges whether the amino group is deprotected by observing the color development of the resin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com