Lactobacillus rhamnosus CCFM1130 and application thereof in relieving and treating gout
A technology of CCFM1130 and Lactobacillus rhamnosus, applied in the field of microbiology, can solve problems such as undiscovered, and achieve the effects of promoting expression, reducing serum uric acid levels, and reducing gout
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Screening of Lactobacillus rhamnosus CCFM1130
[0050] (1) Isolation and screening of Lactobacillus
[0051] (1) Take 1g of fresh feces of healthy adults. After gradient dilution, apply it to LBS medium with 1% nystatin, and place it in a constant temperature incubator at 37°C for 48 hours.
[0052] (2) After cultivation, according to the color, size, and edge shape of the colony, use an inoculation loop to pick up the colony and streak it for purification.
[0053] (3) The obtained colonies were subjected to Gram staining and catalase analysis.
[0054] (4) Retain Gram-positive bacilli and catalase-negative bacteria.
[0055] (2) Molecular biological identification of Lactobacillus
[0056] (1) Single bacterial genome extraction
[0057] (A) cultivating the lactobacilli screened in step (1) overnight;
[0058] (B) Take 1 mL of the bacterial suspension cultivated overnight in a 1.5 mL centrifuge tube, centrifuge at 10000 r / min for 2 min, discard the supern...
Embodiment 2
[0074] Example 2: Detection of tolerance of Lactobacillus rhamnosus CCFM1130 to simulated gastrointestinal fluid
[0075] The cryopreserved Lactobacillus rhamnosus CCFM1130 was inoculated in the MRS medium, cultured at 37°C for 14 hours, and then subcultured in the MRS medium for 2 to 3 times.
[0076] Take 3mL of the culture solution of Lactobacillus rhamnosus CCFM1130 and centrifuge at 8000×g for 2min to collect the bacteria, mix it with 3mL pH 3.0 artificial simulated gastric juice (containing 3g / L pepsin, pH=3.0 physiological saline), and incubate at 37°C. Cultivate, take samples at 0h and 2h respectively, use MRS agar medium to pour culture, count the colonies on the plate, determine the number of viable bacteria and calculate their survival rate.
[0077] Take 3 mL of the culture solution of Lactobacillus rhamnosus CCFM1130 and centrifuge at 8000×g for 2 min to collect the cells, add 3 mL of pH8.0 artificial simulated intestinal fluid (physiological saline containing 1 g...
Embodiment 3
[0081] Example 3: Lactobacillus rhamnosus CCFM1130 has no toxic side effects on KunMing mice
[0082] Suspend Lactobacillus rhamnosus CCFM1130 in 100g / L skim milk solution to make a concentration of 4.0×10 9 CFU / mL bacterial suspension. Twelve healthy male KunMing mice with a body weight of about 24-32 g were taken, and after one week of adaptation to the environment, they were divided into CCFM1130 group and control group. The CCFM1130 group was intragastrically administered 0.3 mL of the bacterial suspension at this concentration once a day, and the control group was intragastrically administered the same volume of 100 g / L skim milk solution without Lactobacillus rhamnosus CCFM1130, observed for one week, and recorded death and body weight.
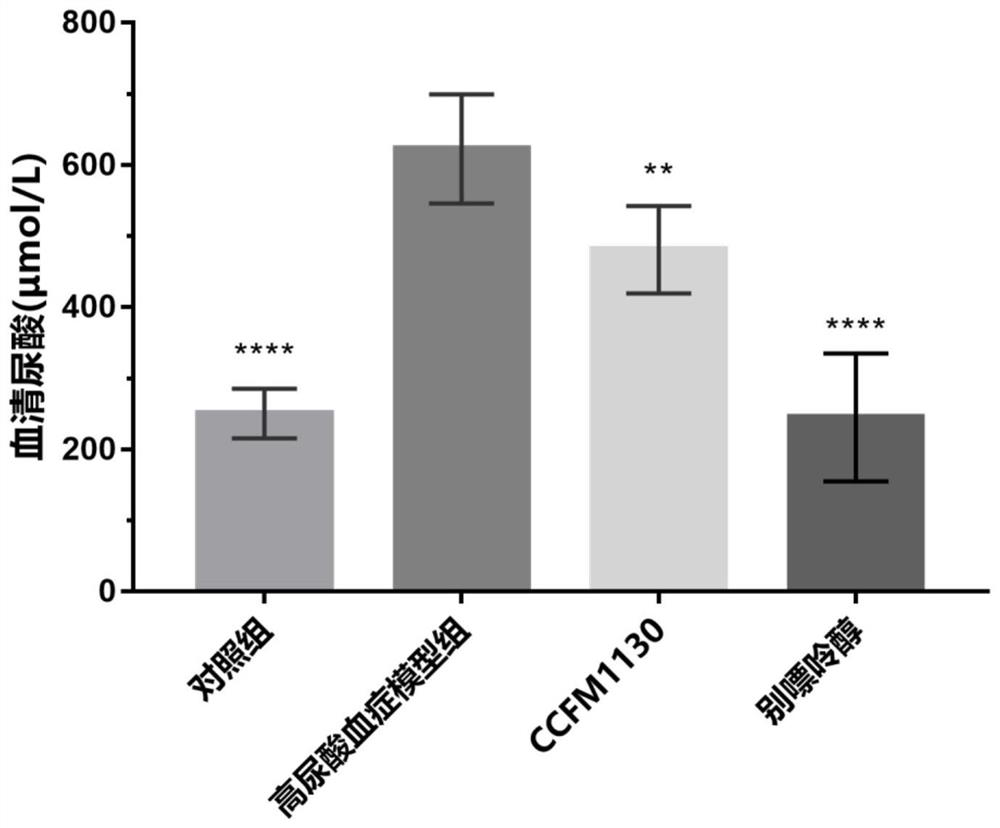
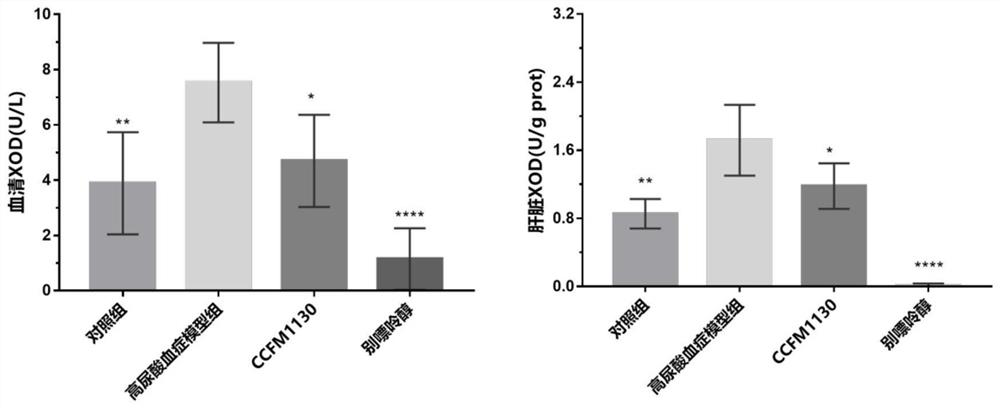
[0083] The results of these tests are listed in Table 2. The results showed that feeding concentration 1×10 9 CFU / Lactobacillus rhamnosus CCFM1130 had no significant impact on mice, no significant change in body weight, and no death....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com