Preparation method of ectoin-hyaluronic acid composite gel and obtained product
A technology of hyaluronic acid and ectoine, applied in the fields of pharmaceutical formulation, pharmaceutical science, organic chemistry, etc., can solve the problems of complex cross-linked gel components, mixed types of cross-linked products, and difficult to control impurity content, etc. Improve immune protection, less impurities, mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
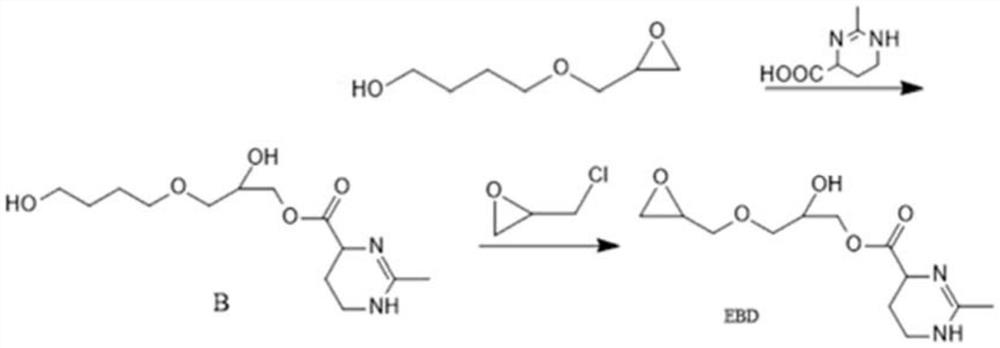
[0042] The preparation of cross-linking agent EBD is as follows:
[0043] (1) Add 18g of 1,4-butanediol and 0.18g of boron trifluoride ethyl ether into the flask, raise the temperature to 50°C, add 9.2g of epichlorohydrin dropwise, continue to react for 4 hours after dropping, and add 100ml after cooling to room temperature Add toluene, stir, then add 10ml of sodium hydroxide (aq, 40%), and continue the reaction at 45°C for 1 hour. After the reaction, 100ml of water was added, extracted with ethyl acetate, and concentrated to obtain a yellow oily product, which was 1,4-butanediol monoglycidyl ether.
[0044] (2) Dissolve 14.6g of 1,4-butanediol monoglycidyl ether and 17.1g of ectoine in 100ml of toluene, add 0.34g of chromium 5-tert-butylfuroate, stir at 115°C for 6 hours, add 50ml of water after the reaction is completed, Ethyl acetate extraction, concentration, column chromatography, the product B was obtained.
[0045] (3) Dissolve 14.4g of product B in toluene, add 0.072...
Embodiment 2
[0047] The preparation of cross-linking agent EBD is as follows:
[0048] (1) Add 18g of 1,4-butanediol and 0.09g of boron trifluoride diethyl ether into the flask, raise the temperature to 40°C, add 7.4g of epichlorohydrin dropwise, continue the reaction for 2 hours after the drop, and add 100ml after cooling to room temperature Add toluene, stir, then add 10ml of sodium hydroxide (aq, 40%), and continue the reaction at 45°C for 1 hour. After the reaction, 100ml of water was added, extracted with ethyl acetate, and concentrated to obtain a yellow oily product, which was 1,4-butanediol monoglycidyl ether.
[0049] (2) Dissolve 14.6g of 1,4-butanediol monoglycidyl ether and 14.2g of ectoine in 100ml of acetone, add 0.071g of chromium 5-tert-butylfuroate, stir at 60°C for 2 hours, add 50ml of water after the reaction is complete, Ethyl acetate extraction, concentration, column chromatography, the product B was obtained.
[0050] (3) Dissolve 14.4g of product B in acetone, add ...
Embodiment 3
[0052] The preparation of cross-linking agent EBD is as follows:
[0053] (1) Add 18g of 1,4-butanediol and 2.7g of boron trifluoride ethyl ether into the flask, raise the temperature to 80°C, add 10.8g of epichlorohydrin dropwise, continue to react for 12 hours after dropping, cool to room temperature and add 100ml of toluene was stirred, and then 10ml of sodium hydroxide (aq, 40%) was added, and the reaction was continued at 45°C for 1 hour. After the reaction, 100ml of water was added, extracted with ethyl acetate, and concentrated to obtain a yellow oily product, which was 1,4-butanediol monoglycidyl ether.
[0054] (2) Dissolve 14.6g of 1,4-butanediol monoglycidyl ether and 28.4g of ectoine in 100ml of n-hexane, add 1.46g of chromium 5-tert-butylfuroate, stir at 80°C for 12h, add 50ml of water after the reaction , extracted with ethyl acetate, concentrated, and column chromatographed to obtain the product B.
[0055] (3) Dissolve 14.4g of product B in n-hexane, add 1.44...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com