Lactobacillus crispatus for preventing and/or treating genital tract flora disorder related diseases
A technology of Lactobacillus crispatus and bacterial suspension, which is applied in the biological field, can solve the problems of drug resistance of pathogenic bacteria, unfavorable reproductive tract maintenance, and reduced abundance of probiotics, etc., to achieve growth ability acid and alkali resistance, reduce recurrence rate, The effect of improving the cure rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Isolation and identification of Lactobacillus crispatus LG55-27
[0051] 1. Sample collection
[0052] The isolated sample comes from the feces of a healthy old man. The sample is collected into a sterile sample tube and brought back to the laboratory for sorting within 1 hour.
[0053] 2. Separation and purification
[0054] The collected fresh samples were immediately transferred to an anaerobic operation box, and 0.2g samples were taken in 1mL sterile PBS (phosphate buffer solution), shaken and mixed thoroughly, and then serially diluted and coated. The medium used was a modified version of PYG medium , the specific formula is (1L): tryptone 8g, soybean peptone 2g, polyprotein 1g, casein 1g, yeast powder 10g, beef extract 5g, glucose 5g, K 2 HPO 4 2g, maltose 0.5g, cellobiose 0.5g, soluble starch 0.5g, sodium sulfide 0.25g, Tween 800.5mL, Cysteine-HCl·H 2 O 0.5g, glycerin 0.5mL, sodium acetate 5g, heme 5mg, vitamin K 11μL, inorganic salt solution (eac...
Embodiment 2
[0068] Example 2: Genome sequencing and species classification and functional gene analysis of Lactobacillus crispatus LG55-27
[0069] 1. Genome sequencing
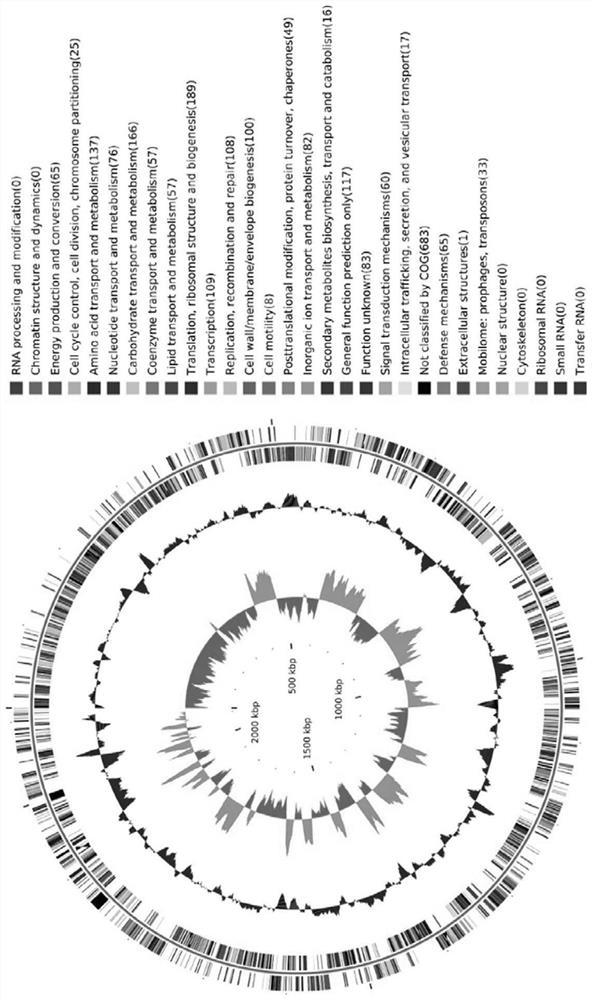
[0070] Centrifuge the overnight cultured LG55-27 bacterial solution at 7,227 g at 4°C for 10 minutes, resuspend the obtained pellet in 1 mL of Tris-EDTA, add 50 μL of 10% SDS and 10 μL of proteinase K (20 mg / mL) at 55°C The cells were lysed in a water bath for 2 hours, and the DNA was extracted by the phenol-chloroform method. And use the Illumina Hiseq 2000 platform to sequence it, the sequencing length is 500bp in both directions, and use SOAPdenovo to assemble the reads. After evaluation, use GCskew to analyze the GC content of the whole genome and visualize the whole genome sequence and functional distribution ( figure 1 ).
[0071] 2. Classification of strain genome species
[0072] Using Checkm software to compare and analyze the whole genome sequence of LG55-27, the most similar species information to this gen...
Embodiment 3
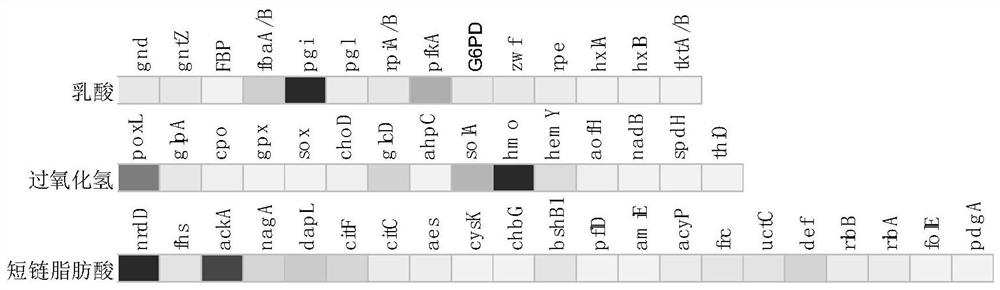
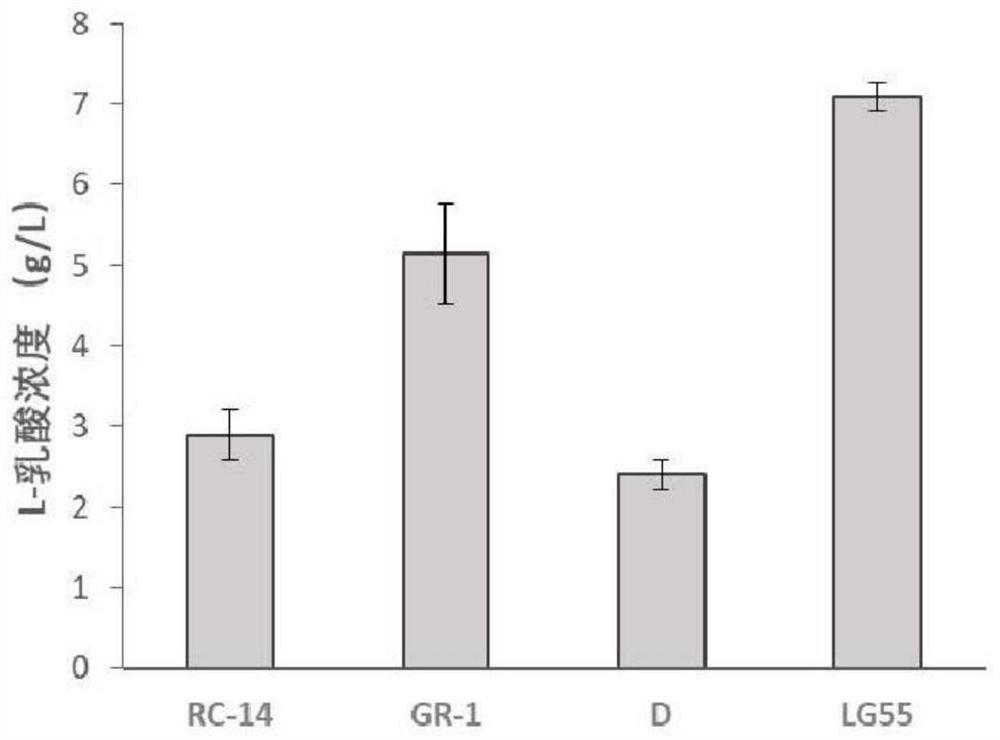
[0075] Embodiment 3: the bioactive substance that Lactobacillus crispatus (Lactobacillus crispatus) LG55-27 produces
[0076] The bioactive substances of LG55-27 mainly examine the content of L-lactic acid and D-lactic acid in the metabolites, and the production of hydrogen peroxide.
[0077] 1. Sample pretreatment
[0078] Strain LG55-27 was inoculated into MRS medium, and cultured at 37°C for 24h under aerobic and anaerobic conditions, respectively.
[0079] Take 1mL of the bacterial solution and centrifuge at 8000r / min for 5min, and take the supernatant to detect the content of L-lactic acid and D-lactic acid.
[0080] Then take 1 mL of the bacterial solution and add lysozyme (final concentration: 1 mg / mL), let it stand at 37°C for 15 min, centrifuge at 8000 r / min for 5 min, take the supernatant, and test the concentration of hydrogen peroxide.
[0081] 2. Measurement method
[0082] The content of L-lactic acid and D-lactic acid is measured by the method in patent CN104...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com