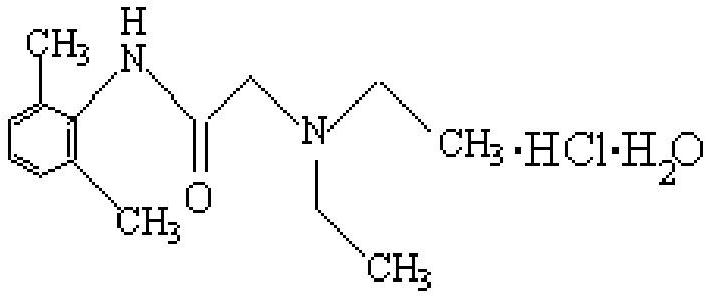
Synthesis method of lidocaine hydrochloride
A technology of lidocaine hydrochloride and its synthetic method, which is applied in the field of drug synthesis, can solve problems such as large environmental pollution, unfavorable green process requirements, high toxicity of chloroacetyl chloride, etc., and achieve the effect of improving yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) According to the mass ratio of 2,6-dimethylaniline: acetonitrile: methyl chloroacetate is 1:4:1, put methyl chloroacetate and acetonitrile into a dry and clean reaction pot, start stirring, and cool to below 20°C , slowly add 2,6-dimethylaniline (control the internal temperature below 20°C), stir and heat up to reflux after the addition, keep the temperature for 3 hours, cool to below 10°C, shake off the material, rinse, spin dry, and dry in Dry at 70°C for 24 hours to obtain intermediate 1; calculated based on the theoretical yield of 2,6-dimethylaniline, the yield of intermediate 1 reaches 95.8%.
[0025] (2) lidocaine base
[0026] According to the mass ratio of intermediate 1, diethylamine and acetonitrile as 1:2.5:5, put intermediate 1, diethylamine and acetonitrile into the reaction kettle, stir and heat up to reflux, keep warm and reflux for 8 hours, and recover acetonitrile after reflux , cooled to 40°C, shaken and filtered, the collected filter residue was...
Embodiment 2
[0031] (1) According to the mass ratio of 2,6-dimethylaniline: acetonitrile: methyl chloroacetate is 1:5:1, put methyl chloroacetate and acetonitrile into a dry and clean reaction pot, start stirring, and cool to below 20°C , slowly add 2,6-dimethylaniline (control the internal temperature below 20°C), stir and heat up to reflux after the addition, keep the temperature for 3 hours, cool to below 10°C, shake off the material, rinse, spin dry, and dry in Dry at 80°C for about 24 hours to obtain intermediate 1; calculated based on the theoretical yield of 2,6-dimethylaniline, the yield of intermediate 1 reaches 96.2%.
[0032] (2) lidocaine base
[0033] According to the mass ratio of intermediate 1, diethylamine and acetonitrile as 1:2.5:5, put intermediate 1, diethylamine and acetonitrile into the reaction kettle, stir and heat up to reflux, keep warm and reflux for 8 hours, and recover acetonitrile after reflux , cooled to 40°C, shaken and filtered, the collected filter resid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com