Application of borane-pyridine complex in preparation of NK-1 receptor antagonist
A receptor antagonist, NK-1 technology, applied in the preparation of organic compounds, the preparation of aminohydroxy compounds, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of unsuitable industrial production, harsh reaction conditions, The technical process is cumbersome and other problems, so as to avoid high pressure environment and the use of heavy metals, mild reaction conditions, and high catalytic efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
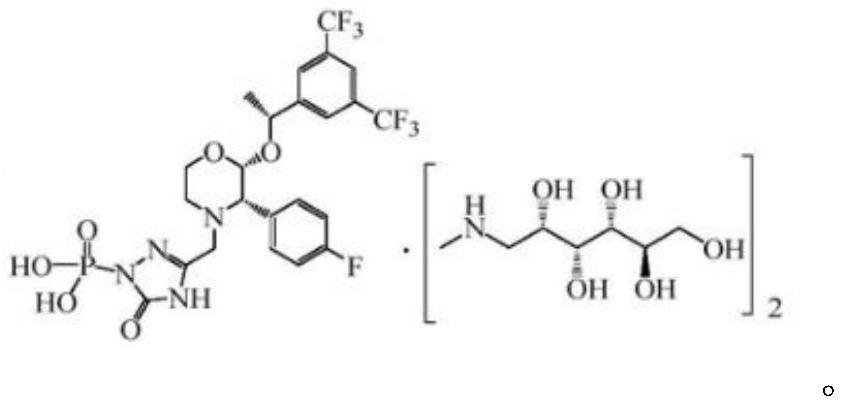
[0029] The invention provides a preparation method of fosaprepitant dimeglumine, which is characterized in that it comprises the following steps: step 1) aprepitant dibenzyl phosphate is catalyzed and prepared under the action of a borane-pyridine complex to obtain intermediate Body fosaprepitant; step 2) fosaprepitant reacts with N-methyl-D-glucosamine again to generate fosaprepitant dimeglumine
[0030] The structural formula of the aprepitant dibenzyl phosphate is: Wherein R is independently selected from a hydrogen atom, a C1-6 alkyl group, a halogen atom, preferably a hydrogen atom.
[0031] According to the preparation method of a kind of NK-1 receptor antagonist fosaprepitant dimeglumine of the present invention, said step 1) comprises: aprepitant dibenzyl phosphate, borane-pyridine complex compound, added in the first organic solvent, stirred and reacted, concentrated under reduced pressure, and recrystallized to obtain the fosaprepitant compound.
[0032] According...
Embodiment 1
[0044] step 1)
[0045] Under a nitrogen atmosphere, add 500ml tetrahydrofuran, 0.1mol aprepitant, and 0.12mol tetrabenzyl pyrophosphate to the reaction flask in sequence, lower the temperature of the system to about 0°C, and dissolve 0.2mol hexamethyldisilazol in tetrahydrofuran Sodium azane was added dropwise into the system, and after the dropwise addition was completed, the reaction was incubated for 1 hour. The reaction was quenched with saturated sodium bicarbonate solution and extracted with methyl tert-butyl ether. The organic layer was washed with 200ml of saturated sodium bisulfate solution, 200ml of saturated sodium bicarbonate solution, and 200ml of saturated sodium chloride solution, dried over anhydrous sodium sulfate, and concentrated to give aprepitant dibenzyl phosphate as a white solid with a yield of 93.6%. .
[0046] step (2)
[0047] Under a nitrogen atmosphere, mix 50 mmol of the product obtained in the previous step with 100 ml of methanol, stir evenl...
Embodiment 2
[0051] step 1)
[0052] Under a nitrogen atmosphere, add 500ml tetrahydrofuran, 0.1mol aprepitant, and 0.12mol tetrabenzyl pyrophosphate to the reaction flask in sequence, lower the temperature of the system to about 5°C, and dissolve 0.2mol hexamethyldisilazol in tetrahydrofuran Sodium azane was added dropwise into the system, and after the dropwise addition was completed, the reaction was incubated for 1 hour. The reaction was quenched with saturated sodium bicarbonate solution and extracted with methyl tert-butyl ether. The organic layer was washed with 200ml of saturated sodium bisulfate solution, 200ml of saturated sodium bicarbonate solution, and 200ml of saturated sodium chloride solution, dried over anhydrous sodium sulfate, and concentrated to give aprepitant dibenzyl phosphate as a white solid with a yield of 93.1%. .
[0053] step (2)
[0054] Under a nitrogen atmosphere, mix 100mmol of the product obtained in the previous step with 180ml of methanol, stir at roo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com