Ulllazine derivatives containing diarylboron and diarylamine and synthesis method thereof
A kind of technology of diarylamine and diarylboron, which is applied to a class of Ullazine derivatives containing diarylboron and diarylamine and its synthesis field, can solve the problem that there is no research report on triarylboron and triarylamine, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
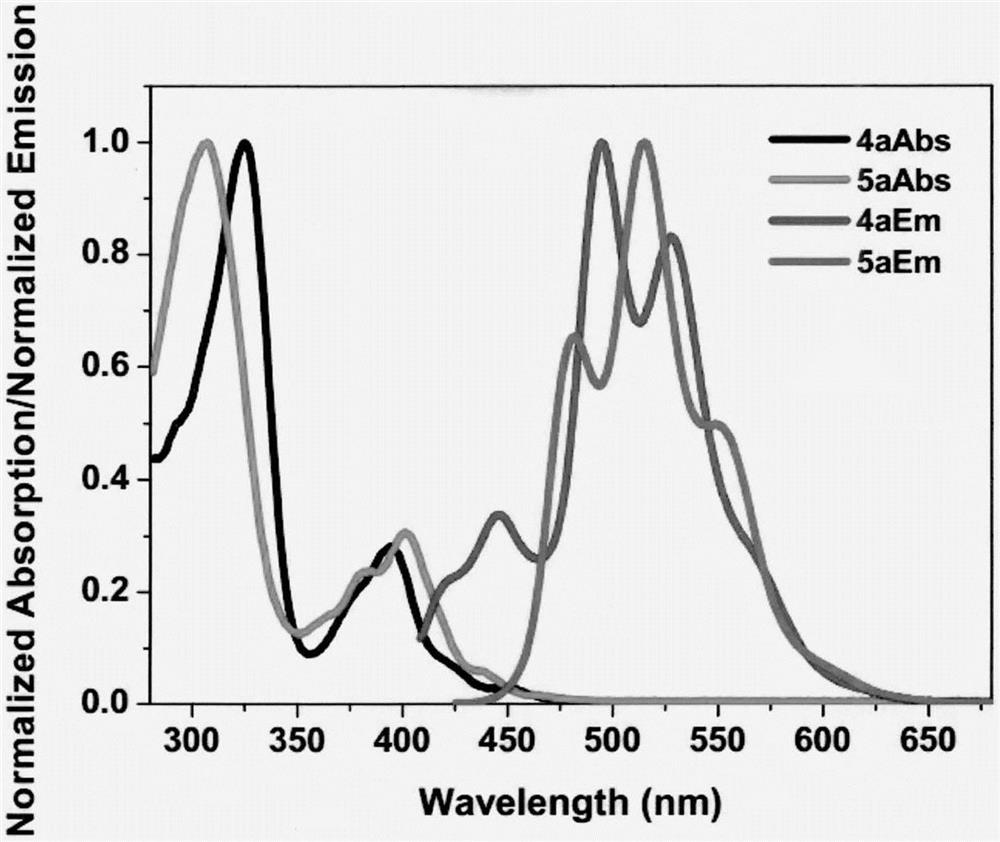
Image
Examples
Embodiment 1
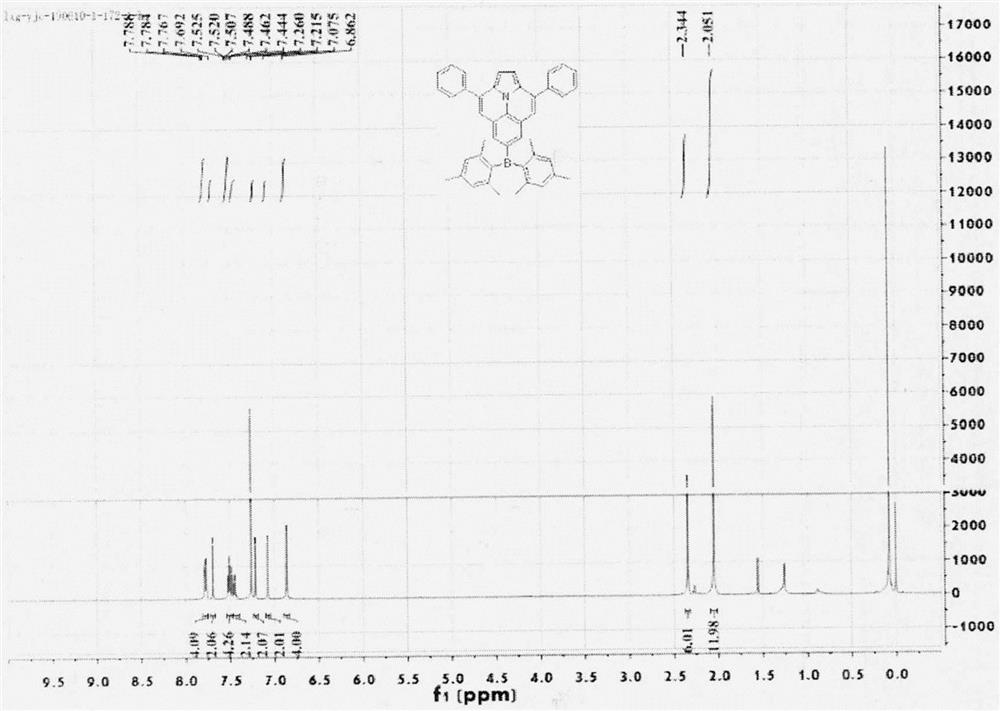
[0039] Embodiment 1: the synthesis of compound 4a
[0040]
[0041] 1) Synthesis of compound 2a: Weigh an appropriate amount of 1-(2,6-diethynylphenyl)-1H-pyrrole, tetrakistriphenylphosphine palladium, cuprous iodide, 1-iodobenzene, nitrogen protection, add Anhydrous triethylamine and tetrahydrofuran were refluxed at 80°C for 24h. After the reaction is complete, filter, spin dry with triethylamine, filter with dichloromethane and water, dry over anhydrous magnesium sulfate, and separate by column chromatography after filtration to obtain the yellow target compound 2a.
[0042] 2) Synthesis of compound 3a: add an appropriate amount of compound 2a to a 50mL test tube, add anhydrous toluene under the protection of anhydrous indium trichloride under nitrogen, and react at 110°C for 24 hours. Extracted with methane and water, the organic phase was dried with anhydrous magnesium sulfate, spin-dried, and purified by column chromatography to obtain compound 3a as a yellow solid. ...
Embodiment 3
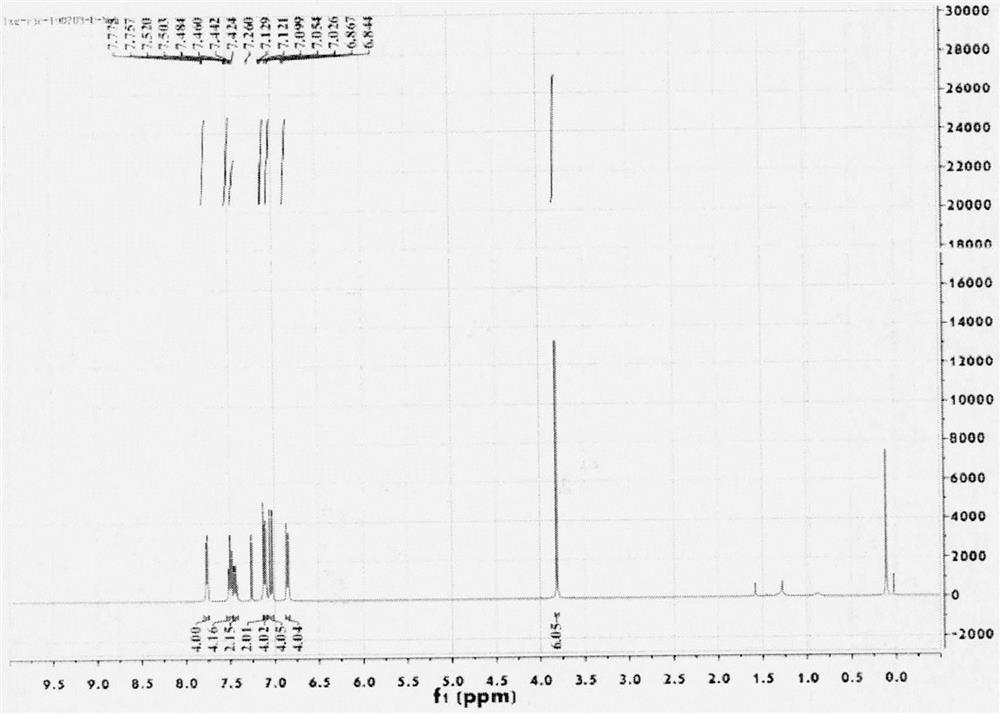
[0051] Embodiment 3: the synthesis of compound 4b
[0052] 1) Synthesis of compound 2b: Weigh an appropriate amount of 1-(2,6-diethynylphenyl)-1H-pyrrole, tetrakistriphenylphosphine palladium, cuprous iodide, (4-iodophenyl) 4- Iodine-N,N-bis(4-methoxyphenyl)aniline was pumped and ventilated 3 times, under nitrogen protection, anhydrous triethylamine was added and refluxed at 80°C for 24h. After the reaction is complete, filter, spin dry with triethylamine, filter with dichloromethane and water, dry over anhydrous magnesium sulfate, and separate by column chromatography after filtration to obtain the yellow target compound 2b.
[0053] 2) Synthesis of compound 3b: add an appropriate amount of compound 2b to a 50mL test tube, add anhydrous toluene under the protection of anhydrous indium trichloride under nitrogen, and react at 110°C for 24 hours. Extracted with methane and water, the organic phase was dried with anhydrous magnesium sulfate, spin-dried, and purified by column c...
Embodiment 4
[0055] Embodiment 4: the synthesis of compound 5b
[0056]
[0057] 1) Synthesis of compound 2b: Weigh an appropriate amount of 1-(2,6-diethynylphenyl)-1H-pyrrole, tetrakistriphenylphosphine palladium, cuprous iodide, (4-iodophenyl) 4- Iodine-N,N-bis(4-methoxyphenyl)aniline was pumped and ventilated 3 times, under nitrogen protection, anhydrous triethylamine was added and refluxed at 80°C for 24h. After the reaction is complete, filter, spin dry with triethylamine, filter with dichloromethane and water, dry over anhydrous magnesium sulfate, and separate by column chromatography after filtration to obtain the yellow target compound 2b.
[0058] 2) Synthesis of compound 3b: add an appropriate amount of compound 2b to a 50mL test tube, add anhydrous toluene under the protection of anhydrous indium trichloride under nitrogen, and react at 110°C for 24 hours. Extracted with methane and water, the organic phase was dried with anhydrous magnesium sulfate, spin-dried, and purified...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com