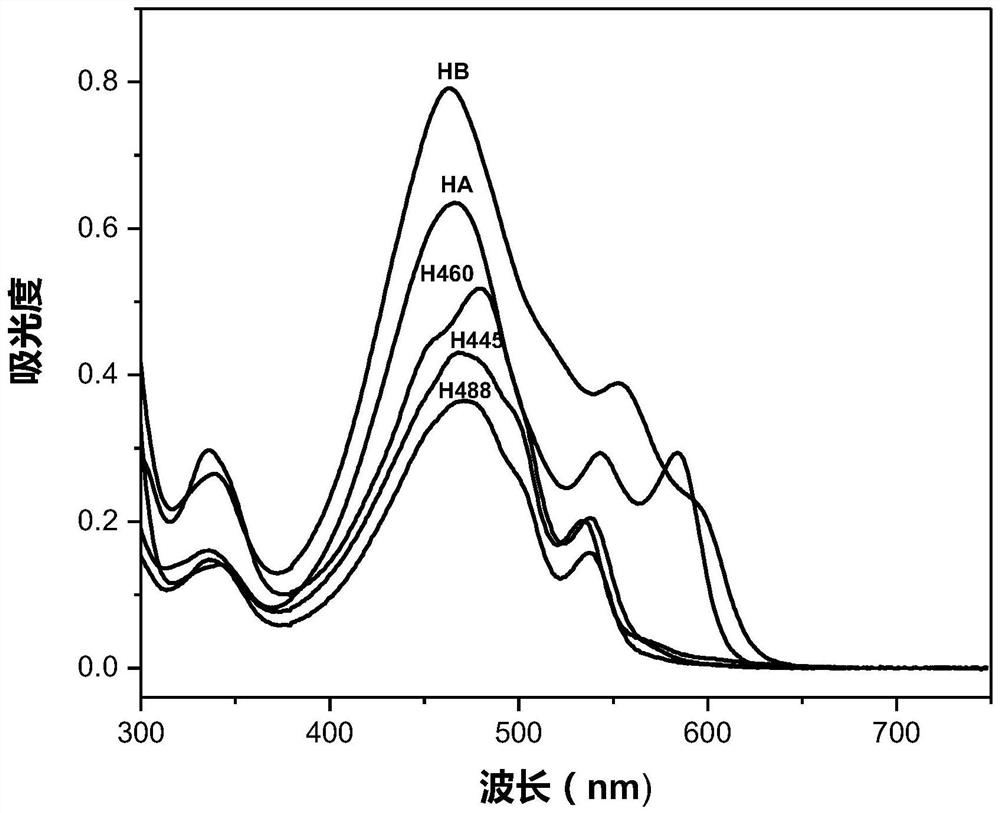
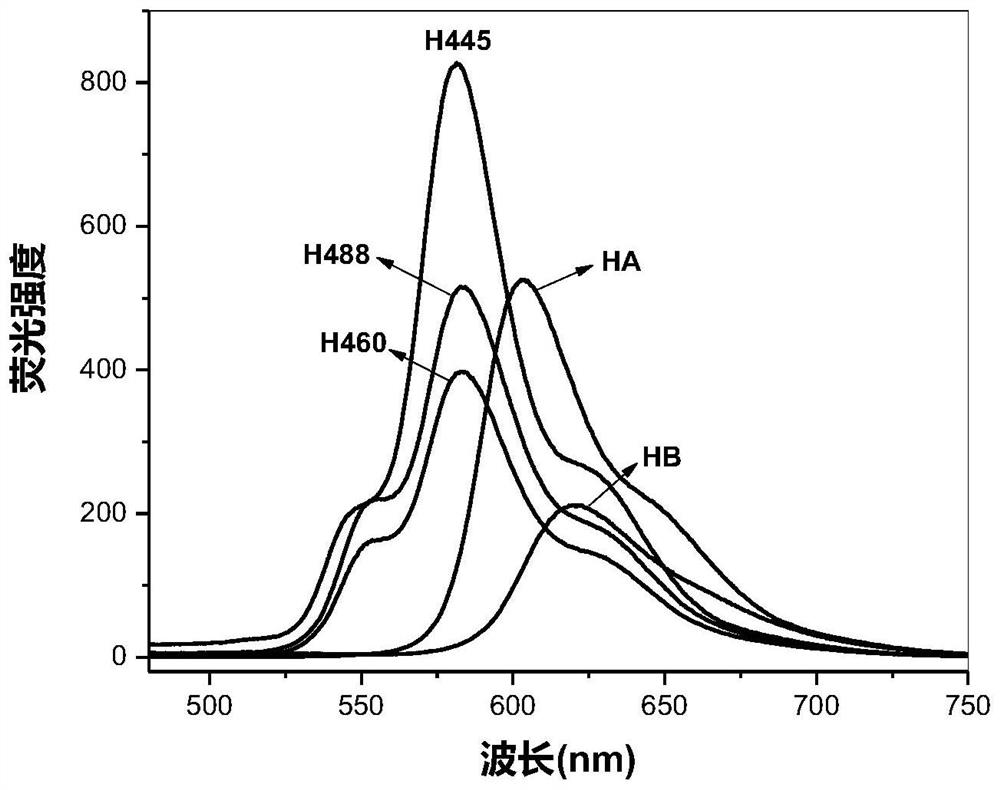
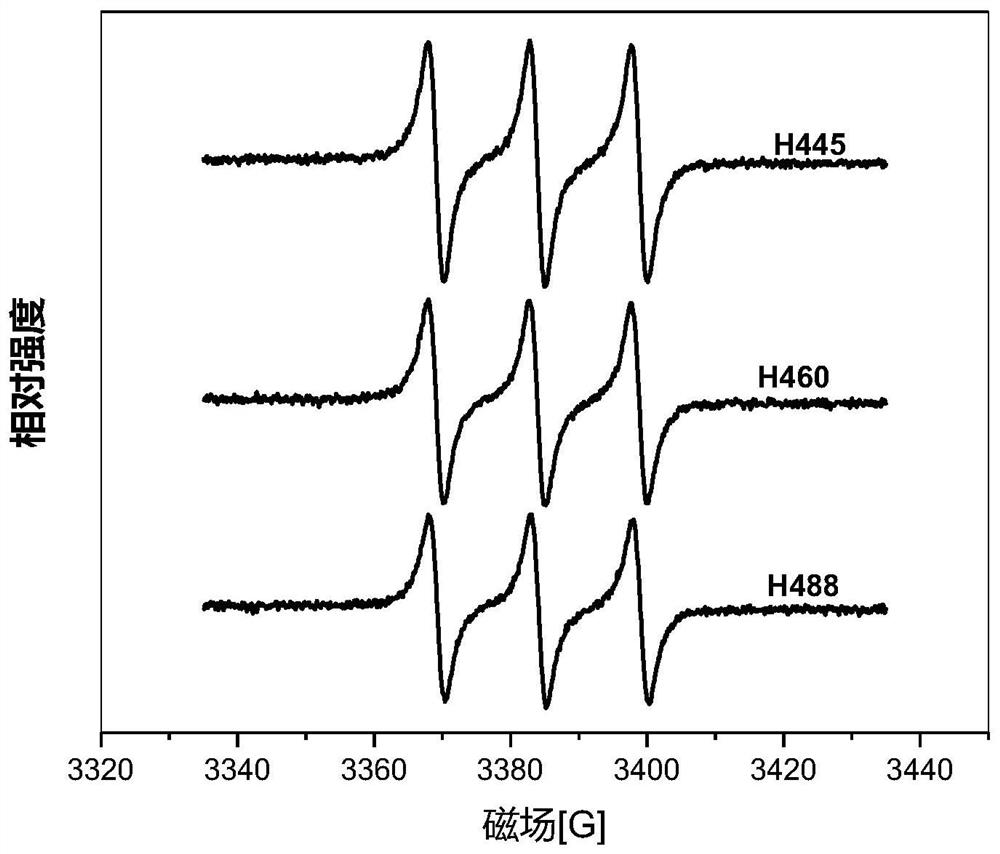
Perylenequinone compound, preparation method and application thereof
A compound and reaction technology, applied in the fields of fluorescence diagnosis and photodynamic medicine, can solve the problems that photosensitizers need to be further improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment 1, preparation Hypocretin 488 (H488)
[0098] 0.60g (4.3×10 -3 mol) potassium carbonate was dissolved in 40.0mL N,N-dimethylformamide (DMF), and Hypocretin A (HA) 25.0mg (4.58×10 -5 mol) was added to the above solution. After fully dissolving, argon gas was introduced, heated at 130°C in the dark, and reacted with electromagnetic stirring for 1.5 hours. After the reaction was completed, the reaction solution was drained under reduced pressure in a water bath at 75°C, neutralized with an appropriate amount of dilute hydrochloric acid, extracted three times with chloroform, washed with water until neutral, and distilled under reduced pressure to obtain an orange powder. Chloroform-petroleum ether (30-60° C.) was recrystallized repeatedly twice to finally obtain 20.8 mg of pure hypocretin 488 (H488) as an orange-red powder with a yield of 93.1%.
[0099] The structural characterization data of this product are as follows:
[0100] UV spectrum λmax (CHCl 3 )...
Embodiment 2
[0111] Embodiment 2, preparation Hypocretin 460 (H460)
[0112] 0.70g (6.6×10 -3 mol) sodium carbonate and 0.12g (8.7×10 -4 mol) potassium oxide was dissolved in 50mL N,N-dimethylformamide (DMF), and Hypocretin B (HB) 40.0mg (7.7×10 -5 mol), added to the above solution. After fully dissolving, argon was introduced, heated at 136°C in the dark, and reacted with electromagnetic stirring for 4.5 hours. After the reaction is completed, drain the reaction solution in a water bath at 75°C under reduced pressure, add 200mL of water, and react with electromagnetic stirring at room temperature in the dark for 16 hours, neutralize with an appropriate amount of dilute hydrochloric acid, extract with dichloromethane three times, wash with water until neutral, and distill under reduced pressure to obtain an orange color powder. Chloroform-petroleum ether (60° C.) was recrystallized several times to obtain 28.9 mg of orange-red powder Hypocretin 460 (H460), with a yield of 83.2%.
[01...
Embodiment 3
[0126] Embodiment 3, preparation Hypocretin 445 (H445)
[0127] 3.0g (2.2×10 -2 mol) potassium carbonate was dissolved in 60mL N,N-dimethylformamide (DMF), and Hypocretin A (HA) 44.0mg (8.1×10 -5 mol) was added to the above solution. After thorough mixing, argon was introduced, heated at 151°C in the dark, and reacted with electromagnetic stirring for 7.5 hours. The reaction solution was drained under reduced pressure in a water bath at 75°C, neutralized with an appropriate amount of dilute hydrochloric acid, extracted several times with chloroform, the combined extracts were washed with water until neutral, and an orange powder was obtained by distillation under reduced pressure. Repeated recrystallization from acetone-petroleum ether (30-60° C.) finally obtained 30.0 mg of orange-red powder hypocretin 445 (H445), with a yield of 83.6%.
[0128] The structural characterization data of this product are as follows:
[0129] UV spectrum λmax (CHCl 3 ): 336.5nm, 468.5nm, 534...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass spectrometry | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com