Detection method of (3R, 4R)-1-benzyl-N, 4-dimethylpiperidine-3-amine dihydrochloride and isomer thereof
A technology of dimethylpiperidine and amine dihydrochloride, which is applied in the field of analytical chemistry, can solve problems that are difficult to achieve, and achieve the effect of ensuring product quality and patient drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
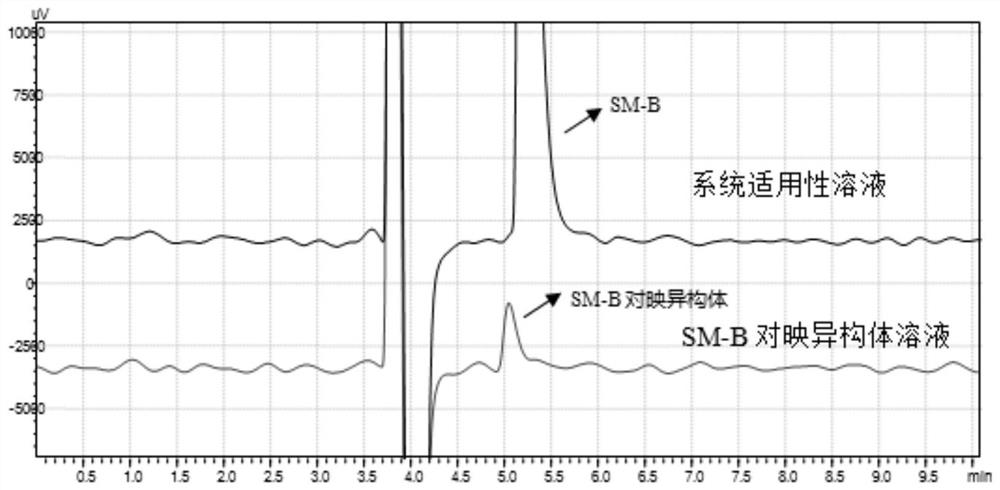
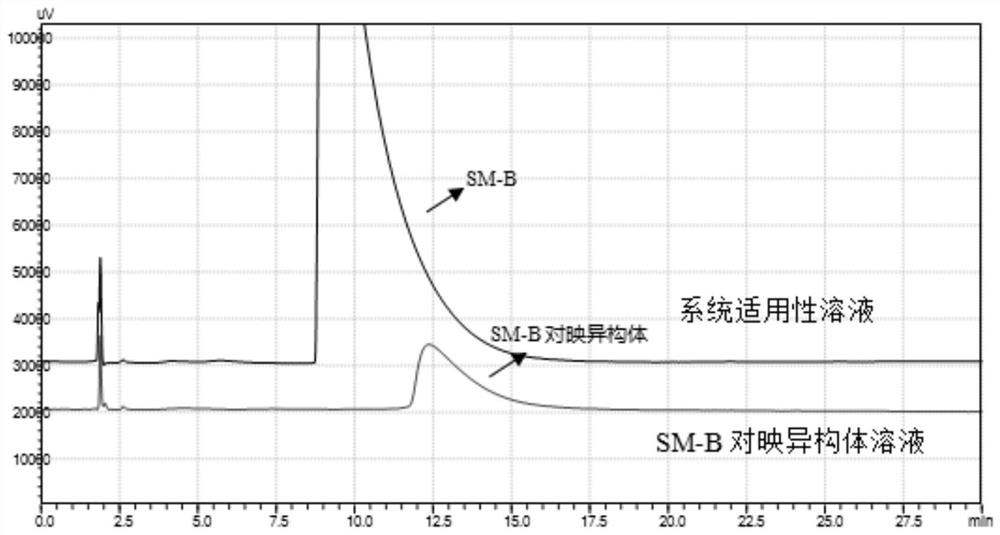
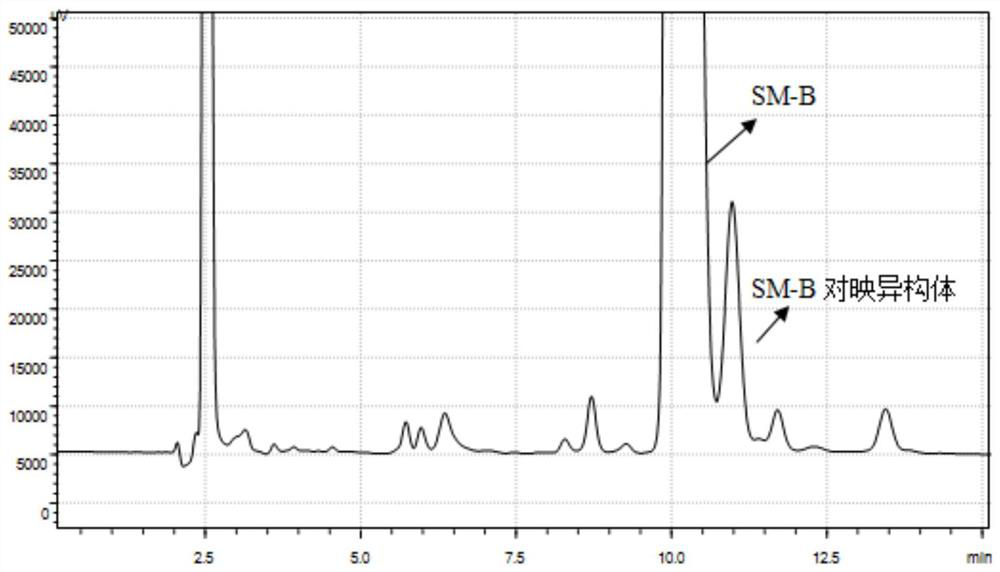
[0029] Example 1 Conventional HPLC chiral column for the separation of SM-B and its enantiomers
Embodiment 2
[0045] The investigation of embodiment 2 different chiral derivatization reagents
[0046] Test Example 1: Using (S)-(+)-α-methoxy-α-(trifluoromethyl)phenylacetyl chloride as derivatization reagent
[0047] Experimental equipment: Aglent 1260 high performance liquid chromatography.
[0048] Chromatographic conditions: the chromatographic column is Welch Ultimate XB-C18 (4.6*250mm, 5μm); the column temperature is 40°C; the ultraviolet detector is used, and the detection wavelength is 210nm; Potassium dihydrogen buffer solution (pH3.0)-methanol (50:50) was used as the mobile phase, and was collected isocratically for 60 minutes.
[0049] Experimental steps:
[0050] 0.1% triethylamine solution: take 100 μl of triethylamine, dilute to 100ml with acetonitrile, and shake well to obtain.
[0051] Derivatization reagent diluent: take 10 mg of (S)-(+)-α-methoxy-α-(trifluoromethyl)phenylacetyl chloride, dilute to 10 ml with acetonitrile, and shake well to obtain.
[0052] SM-B enan...
Embodiment 3
[0073] Embodiment 3 The screening of detection method mobile phase of the present invention
[0074] Test Example 1: Screening of mobile phase A pH value
[0075] The purpose of the experiment: choose 0.01mol / L potassium dihydrogen phosphate aqueous solution as the water phase, adjust different pH values, investigate the influence of mobile phase pH value on the detection of SM-B enantiomers, and select appropriate conditions for SM-B Enantiomer detection.
[0076] Experimental equipment: Shimadzu LC-20AT high performance liquid chromatography.
[0077] Chromatographic conditions: the chromatographic column is Welch Ultimate XB-C18 (4.6*250mm, 5μm); the column temperature is 40°C; the ultraviolet detector is used, and the detection wavelength is 210nm; Potassium dihydrogen buffer solution was mobile phase A, adjusted to three different pH values (respectively pH2.3, pH3.0, pH6.0), and mobile phase A-methanol (50:50) was used for isocratic collection for 60 min.
[0078] E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com