Transparent polyimide film and preparation method thereof
A technology of transparent polyimide and film, applied in the field of flexible display, to achieve the effect of increasing packing density, reducing thermal expansion coefficient and high strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0065] The present invention also provides a kind of preparation method of transparent polyimide film, comprises the following steps:
[0066] The diamine monomer and the dianhydride monomer are condensed and then imidized to obtain a transparent polyimide film.
[0067] Specifically, in the present invention, firstly, the diamine monomer, the dianhydride monomer and a solvent are mixed for condensation reaction to obtain a polyamic acid intermediate.
[0068] In the present invention, the method for obtaining the polyamic acid intermediate is not particularly limited, and it can be synthesized by methods known to those skilled in the art.
[0069] In the present invention, polyamic acid is obtained from monomeric dianhydride and monomeric diamine through the preparation method of polyimide solution polymerization well known to those skilled in the art. That is, mixing dianhydride monomers and diamine monomers in an organic solvent to react. In some specific embodiments of t...
Synthetic example 1
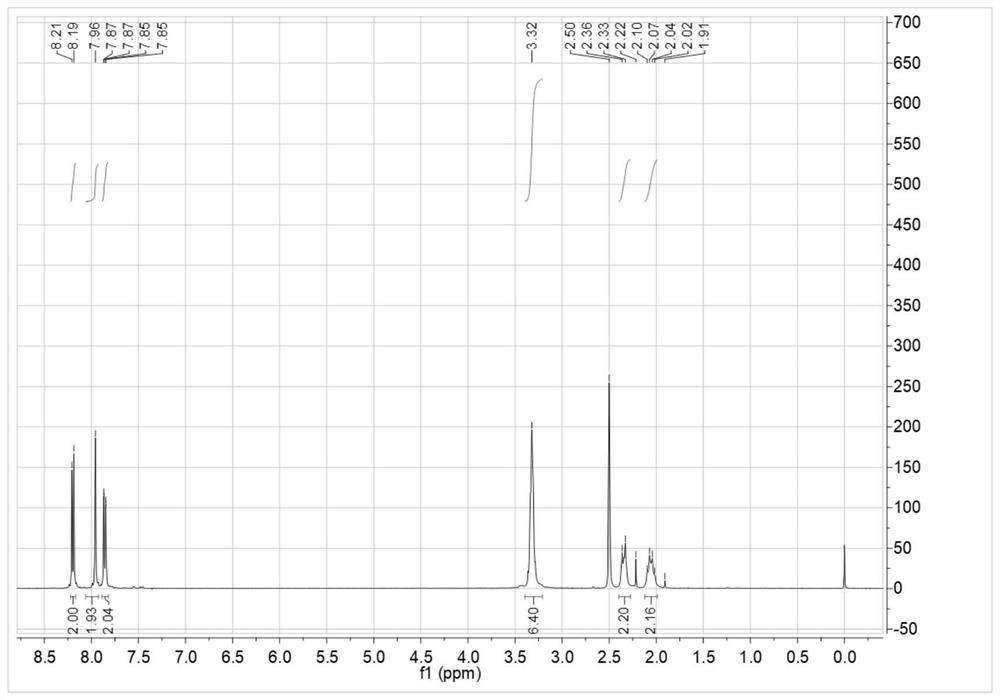
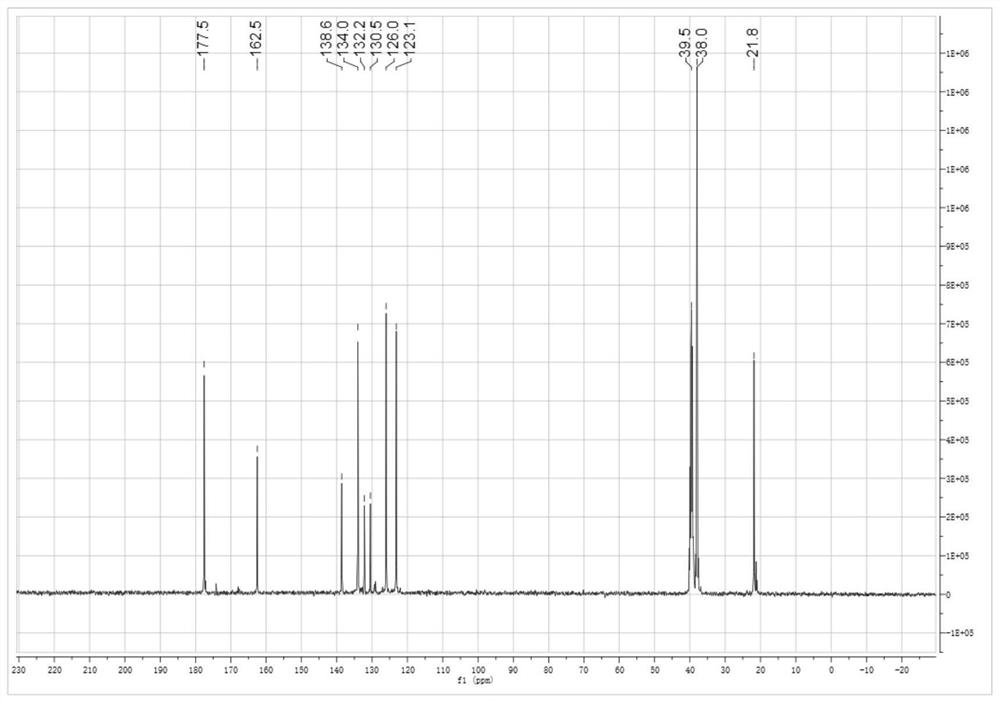
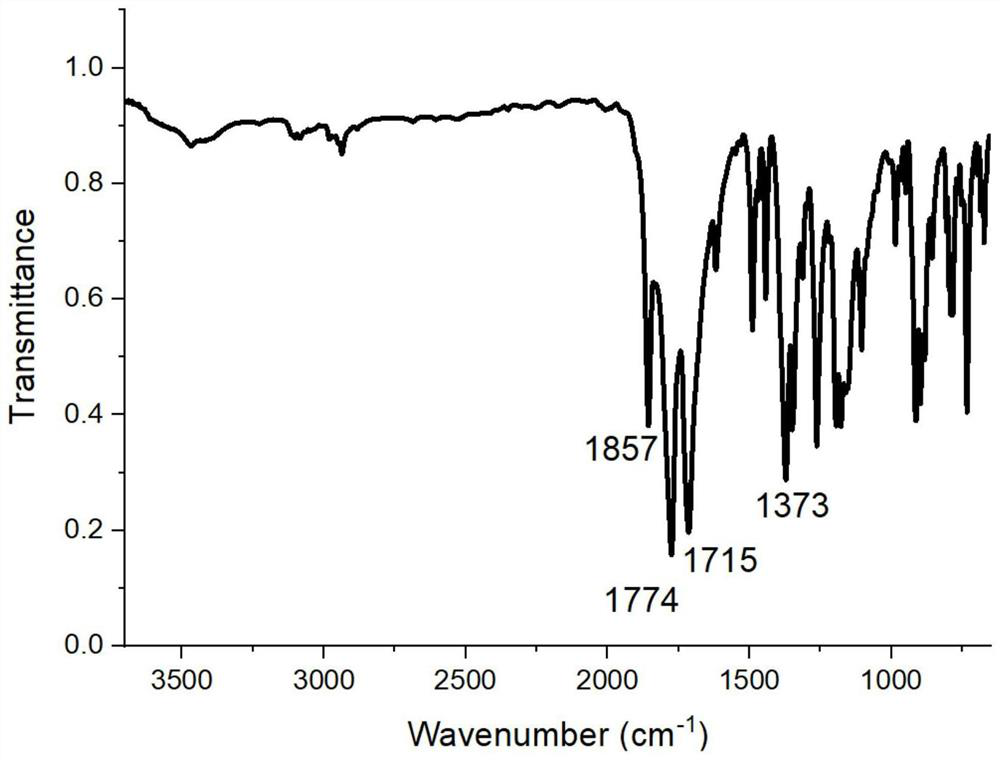
[0110] Add 3.623g of 4-aminophthalic acid and 80g of tetrahydrofuran into the reaction vessel, stir, and add 2.242g of cyclohexanetetracarboxylic dianhydride in batches at 10°C, and react for 15 hours. Filter and filter out the solid powder to obtain the tetraacid after drying. Add the obtained tetraacid into 100g of xylene, heat to reflux with water until the distilled water reaches the theoretical amount, cool, filter, and dry to obtain 5.00g of semi-fatty dianhydride (A1), with a yield of 97.2%. 1 HNMR (400MHz, DMSO-d 6 )δ: 8.20 (d, J = 8.1Hz, 2H), 7.96 (s, 2H), 7.86 (dd, J = 8.1, 1.2Hz, 2H), 3.39–3.21 (m, 4H), 2.34 (d, J =13.2Hz,2H),2.06(dd,J=22.2,9.6Hz,2H). 13 C NMR (101MHz, DMSO-d 6 )δ177.5, 162.5, 138.6, 134.0, 132.2, 130.5, 126.0, 123.1, 39.5, 38.0, 21.9.
[0111]
Synthetic example 2
[0113] Add 3.623g of 4-aminophthalic acid and 100g of acetonitrile into the reaction vessel, stir, and at 10°C, add 3.063g of bicyclohexanetetracarboxylic dianhydride in batches, and react for 12 hours. Filter and filter out the solid powder to obtain the tetraacid after drying. Add the obtained tetra-acid to 120g of cyclohexanone, and heat to reflux with water for 12h. Cool, filter, and dry to obtain 5.66 g of semi-fatty dianhydride (A2), with a yield of 95.0%. 1 HNMR (300MHz, DMSO-d 6 )δ:8.66(s,2H),8.40(d,J=7.5Hz,2H),7.72(d,J=7.5Hz,2H),2.60(m,2H),1.87(m,4H),1.42( m,2H),1.52(m,4H),1.27(m,4H). 13 C NMR (101MHz, DMSO-d 6 )δ176.7, 162.3, 140.4, 132.2, 129.8, 129.5, 126.7, 119.8, 37.7, 36.2, 35.5, 30.4, 28.2, 24.8.
[0114]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com