Cardiovascular and cerebrovascular and diabetes related four-high index composite quality control product and preparation method thereof
A technology for cardiovascular and cerebrovascular and quality control products, applied in the field of medical diagnosis, to achieve the effect of long storage time, ensuring consistency and improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Preparation of composite quality control product of the present invention
[0035] Take 1L of healthy mixed human plasma and place it at 37 degrees to thaw, add 10 mL of protamine sulfate with a concentration of 2-10 mg / mL, stir at 37 degrees for 30 minutes, incubate at room temperature for 2 hours, and then place it at 2-8 degrees for 40 hours to promote fibrin The original degradation product is formed, centrifuged at 10,000rpm for 15min, retaining the supernatant, and filtering with a 0.22μm filter membrane, and the filtered liquid is the matrix fluid with four high indicators.
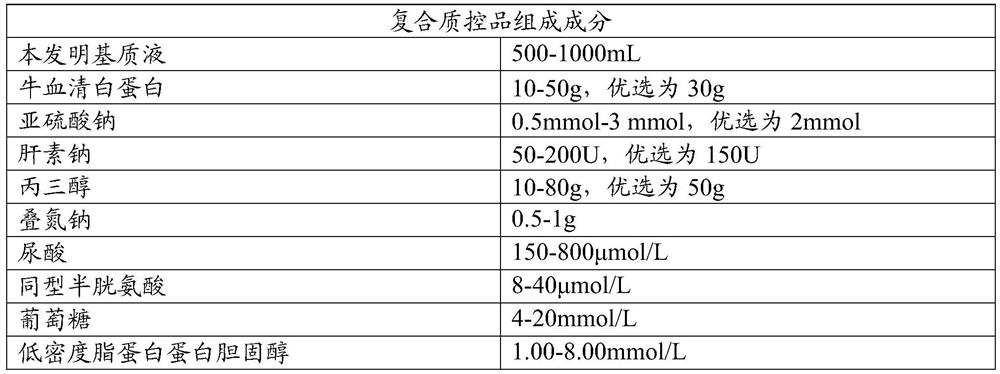
[0036] Sodium sulfite, bovine serum albumin, sodium heparin, glycerol, uric acid, homocysteine, glucose, low-density lipoprotein cholesterol and preservatives were added to the matrix solution after treatment. The specific composition is shown in Table 1 below.
[0037] Table 1
[0038]
Embodiment 2
[0039] Example 2: Effects of different anticoagulants, reducing agents and polyols on the stability of composite quality control products
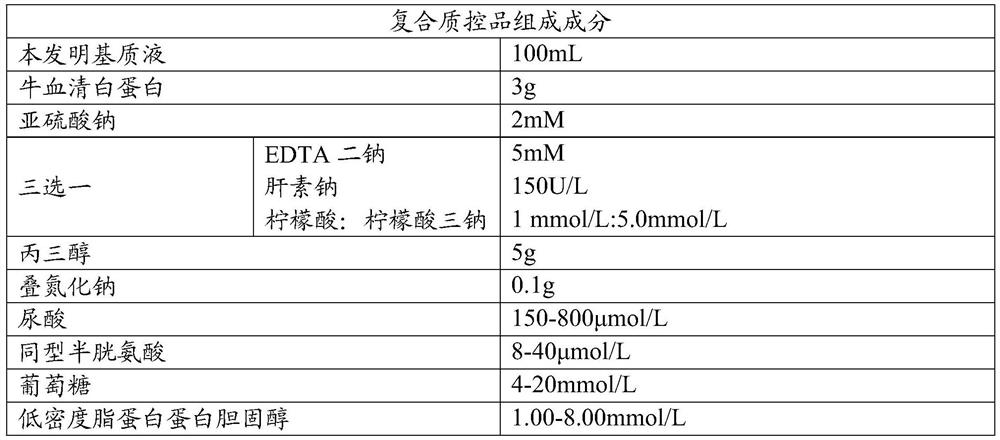
[0040] The mixture of disodium EDTA, citric acid and trisodium citrate, which belong to the same anticoagulant as sodium heparin, was selected as the control; Mannitol and sorbitol, which belong to polyols, were used as controls, and the stability comparison of composite quality control products was carried out under the condition that other conditions remained unchanged;
[0041] 1. The influence of different anticoagulants
[0042] Table 2
[0043]
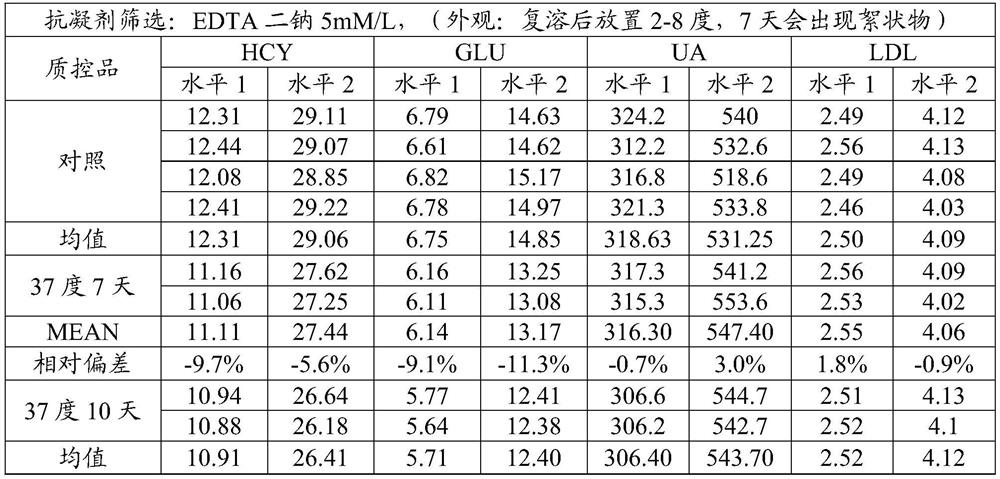
[0044] Prepare three composite quality control products according to the above table 2. After freeze-drying, test the accelerated stability at 37 degrees and the stability of reconstitution and storage in a refrigerator at 2-8 degrees for different days, and test the stability of Glu, Hcy, UA, and LDL-C and observe the appearance after reconstitution, the results are shown in Table 3; ...
Embodiment 3
[0068] Embodiment 3: Comparison of matrix effects of different matrix fluids
[0069] The matrix solutions of quality control products sold in the market are mainly buffer matrix and human blood matrix. In this example, the influence of different matrix solutions on the stability of composite quality control products is compared according to the grouping in Table 8. The results are shown in Table 9.
[0070] Table 8
[0071]
[0072]
[0073] The accelerated stability result of table 9 matrix liquid screening
[0074]
[0075]
[0076] It can be seen from Table 9 that the buffer solution and human serum matrix were used to prepare four high-quality control products, and the matrix was quite different from the clinical samples. The lyophilized powder was accelerated at 37 degrees for 7 days, and the glucose concentration decreased significantly; the stability was poor; However, the matrix solution of the present invention ensures that the matrix is consistent wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com