Economic and safe synthesis method of zero-valent nickel coordination compound
A coordination compound and synthesis method technology, applied in the field of synthesis of zero-valent nickel coordination compounds, can solve the problems of expensive raw materials, unfavorable industrialization, high risk factor, etc., and achieve high product yield and purity, safe and stable reaction, and dangerous reaction small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
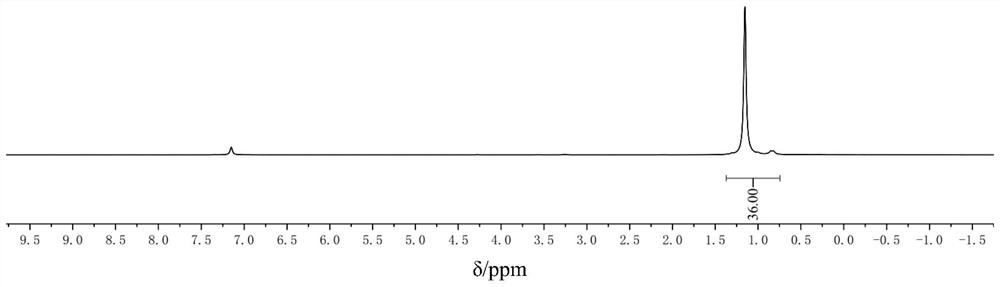
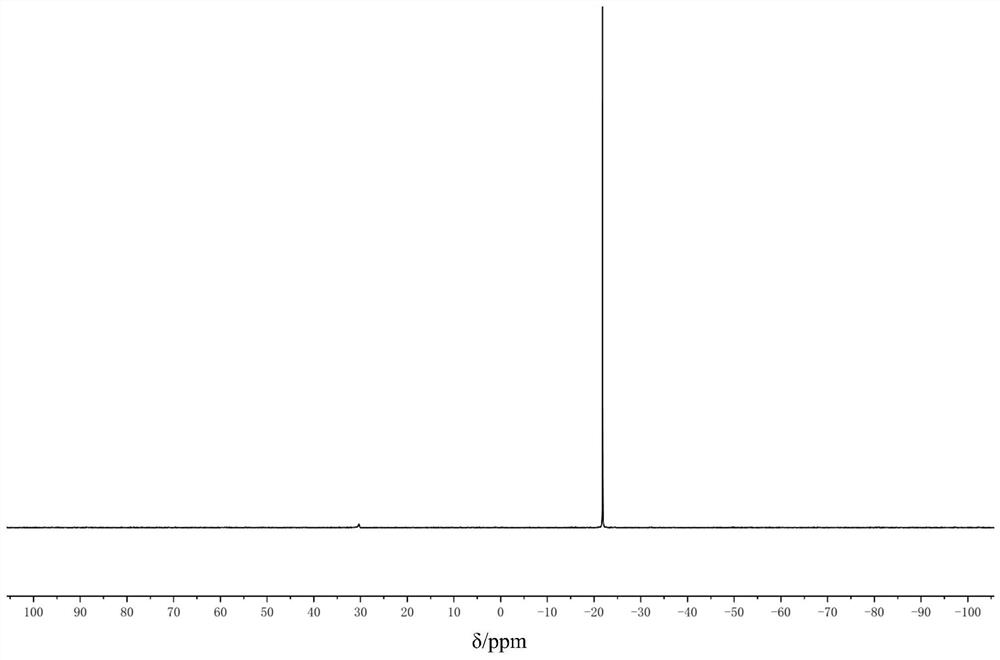
[0020] Add 0.05 mol of nickel chloride hexahydrate into a 250 ml three-neck flask, add a polytetrafluoroethylene magnetic stirring bar, add 0.2 mol of trimethylphosphine, and add 50 ml of tetrahydrofuran. React at room temperature under argon protection, stir for 4 hours, add 6 g of dry molecular sieves, raise the temperature to 50°C and stir for 30 minutes, stir to return to room temperature, add 0.025mol zinc powder, and react for 1 hour. Under the protection of argon, add an appropriate amount of diatomaceous earth dried at 100°C to the funnel, and filter it. The filtrate is vacuum-removed, washed with an appropriate amount of methanol, recrystallized by n-hexane, filtered, and the solid is vacuum-dried to obtain 16.5 g of yellow The solid, ie tetrakis(trimethylphosphine)nickel, has a yield of 91% and a purity of 99%.
[0021] The resulting product is used as a proton nuclear magnetic resonance spectrum and a phosphorus nuclear magnetic resonance spectrum, and the spectrogr...
Embodiment 2
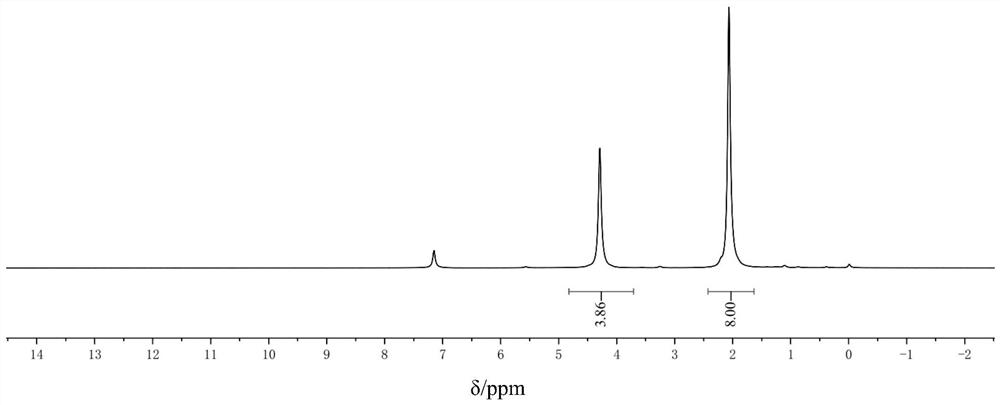
[0023] Add 0.05mol nickel chloride hexahydrate into a 250ml three-necked flask, add a polytetrafluoroethylene magnetic stirring bar, add 0.1mol 1,5-cyclooctadiene, and add 50ml tetrahydrofuran. Reaction at room temperature, protected by argon, stirring for 4.5 hours, adding 6g of dry molecular sieves, raising the temperature to 40°C and stirring for 40 minutes, stirring to return to room temperature, adding 0.025mol zinc powder, and reacting for 1.5 hours. Under the protection of argon, add an appropriate amount of diatomaceous earth dried at 100°C to the funnel, filter, remove the solvent in the filtrate, wash with an appropriate amount of methanol, recrystallize through n-hexane, filter, and dry the solid in vacuum to obtain 12.7 g of Yellow solid, ie bis(1,5-cyclooctadiene)nickel, with a yield of 92.4% and a purity of 99%.
[0024] Gained product is done proton nuclear magnetic resonance spectrum, spectrogram sees image 3 , H1 NMR (400MHz, C 6 D. 6 ): δ4.30(s,8H,8CH),2....
Embodiment 3
[0026] Add 0.05 mol of nickel chloride hexahydrate into a 250 ml three-necked flask, add a polytetrafluoroethylene magnetic stirring bar, add 0.2 mol of triphenylphosphine, and add 50 ml of tetrahydrofuran. Reaction at room temperature, protected by argon, stirring for 5 hours, adding 6 g of dry molecular sieves, raising the temperature to 80°C and stirring for 20 minutes, stirring to return to room temperature, adding 0.025mol zinc powder, and reacting for 2 hours. Under the protection of argon, add an appropriate amount of diatomaceous earth dried at 100°C to the funnel, and filter it. The filtrate is vacuum-removed, washed with an appropriate amount of methanol, recrystallized by n-hexane, filtered, and the solid is vacuum-dried to obtain 51 g of yellow-brown The solid, ie tetrakis(trimethylphosphine)nickel, has a yield of 92% and a purity of 99%.
[0027] The resulting product was subjected to Elemental analysis: C: 78.04, H: 5.48, Ni: 5.36, P: 11.12.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com