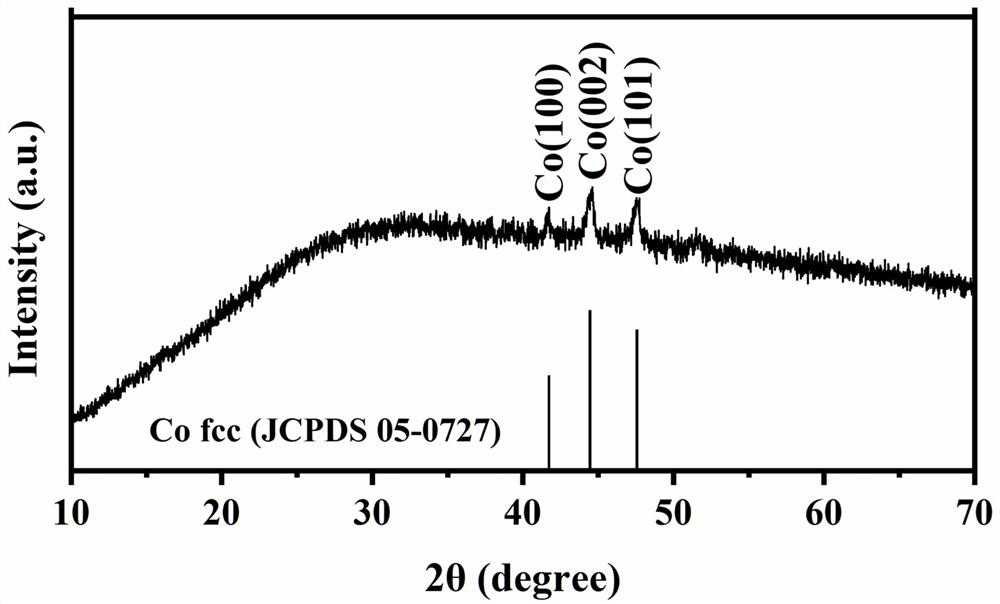
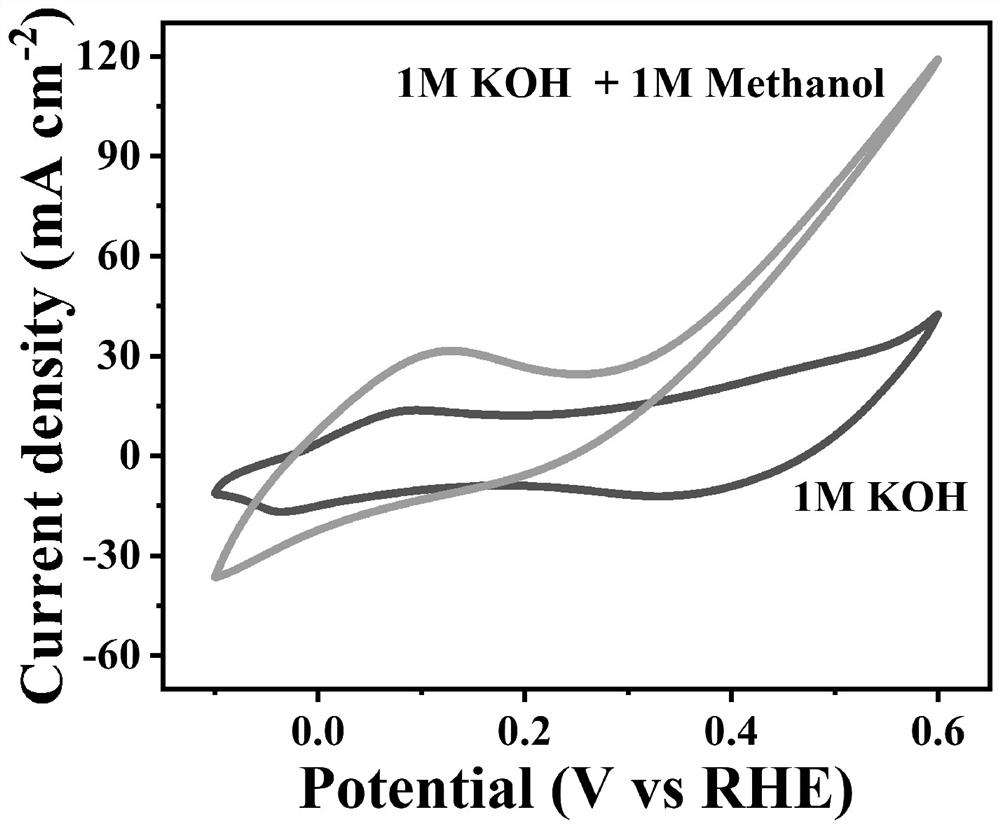
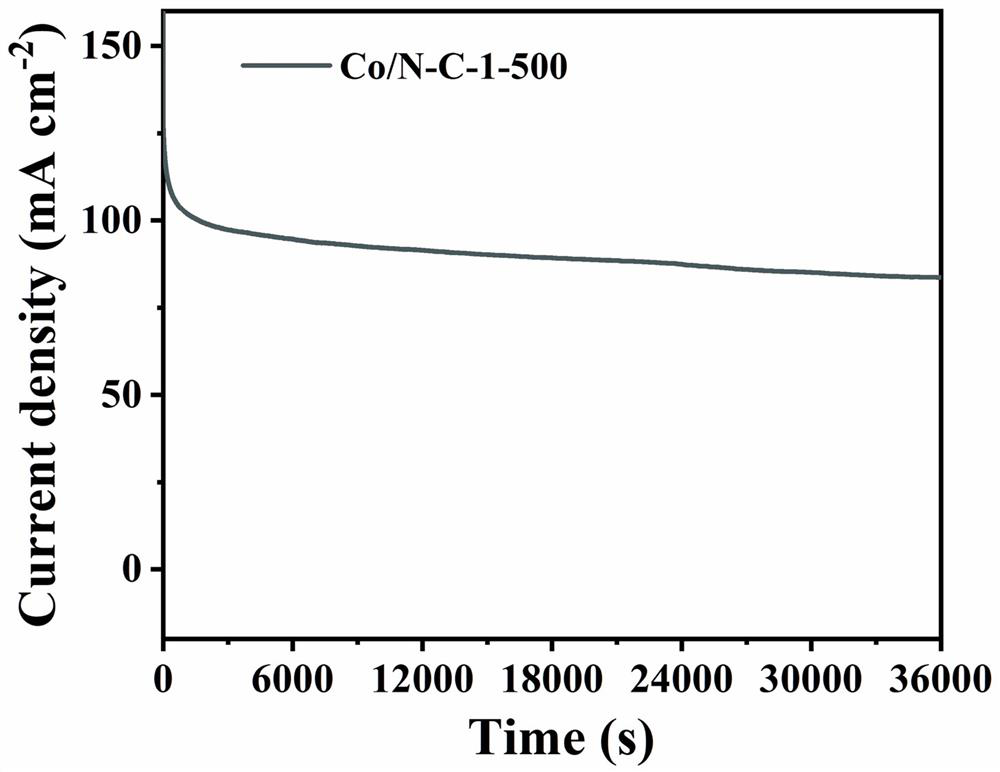
Cobalt-based complex catalyst for direct methanol fuel cell anode, and preparation method thereof
A methanol fuel cell and composite technology, applied in fuel cells, battery electrodes, electrochemical generators, etc., can solve the problems of affecting the electrochemical performance of methanol oxidation, restricting wide application, expensive raw materials, etc., and achieve excellent electrochemical reactivity , Obvious catalytic effect, the effect of improving catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Weigh 0.0303g (0.175mmol) of triaminophenyl borate hydrochloride (ABA), and dissolve it in 835µL of 6mol / L hydrochloric acid solution to prepare an ABA solution.
[0031]Add the above-mentioned ABA solution, 0.233g (2.5mmol) aniline (AN) and 62µL deionized water to 2mL of 10wt% polyvinyl alcohol (PVA) solution in sequence, stir for 30min under heating in a water bath at 55°C, until the solution is clear, place on ice In a water bath, cool to 0°C, add 1.375mL 2mol / L ammonium persulfate (APS) solution dropwise, stir quickly and evenly, pour it into a mold, and polymerize at room temperature for 12 hours to prepare polyvinyl alcohol-polyaniline conductive hydrogel Polymer film PPH.
[0032] Soak PPH in 1mol / L ammonia water until the film turns dark purple, take it out and wash it with deionized water, soak it in 1mol / L cobalt chloride solution for 24 hours, take it out and wash it with deionized water, Freeze-dried for 10 h, placed in a tube reactor, under N 2 Under the ...
Embodiment 2
[0036] Weigh 0.0243g (0.14mmol) of triaminophenyl borate hydrochloride (ABA), and dissolve it in 835µL of 6mol / L hydrochloric acid solution to prepare an ABA solution.
[0037] Add the above-mentioned ABA solution, 0.175g (1.9mmol) aniline (AN) and 62µL deionized water to 2mL 10wt% polyvinyl alcohol (PVA) solution in turn, stir for 30min under heating in a water bath at 55°C, until the solution is clear, place on ice In a water bath, cool to 0°C, add 1.5mL 2mol / L ammonium persulfate (APS) solution dropwise, stir quickly and evenly, pour into a mold, and polymerize at room temperature for 12 hours to prepare polyvinyl alcohol-polyaniline conductive hydrogel Polymer film PPH.
[0038] Soak PPH in 2mol / L ammonia water until the film turns dark purple, take it out and wash it with deionized water, soak it in 1mol / L cobalt chloride solution for 24 hours, take it out and wash it with deionized water, Freeze-dried for 10 h, placed in a tube reactor, under N 2 Under the atmosphere, ...
Embodiment 3
[0041] Weigh 0.0358g (0.21mmol) of triaminophenyl borate hydrochloride (ABA), and dissolve it in 835µL of 6mol / L hydrochloric acid solution to prepare an ABA solution.
[0042] Add the above-mentioned ABA solution, 0.264g (2.8mmol) aniline (AN) and 234µL deionized water to 2mL of 10wt% polyvinyl alcohol (PVA) solution in sequence, stir for 30min under heating in a water bath at 55°C, until the solution is clear, place on ice In a water bath, cool to 0°C, add 1.652mL 2mol / L ammonium persulfate (APS) solution dropwise, stir quickly and evenly, pour into a mold, and polymerize at room temperature for 12 hours to prepare polyvinyl alcohol-polyaniline conductive hydrogel Polymer film PPH.
[0043] Soak PPH in 0.5mol / L ammonia water until the film turns dark purple, take it out and wash it with deionized water, soak it in 2mol / L cobalt chloride solution for 24h, take it out and wash it with deionized water, Freeze-dried for 10 h, placed in a tube reactor, under N 2 Under the atmos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com