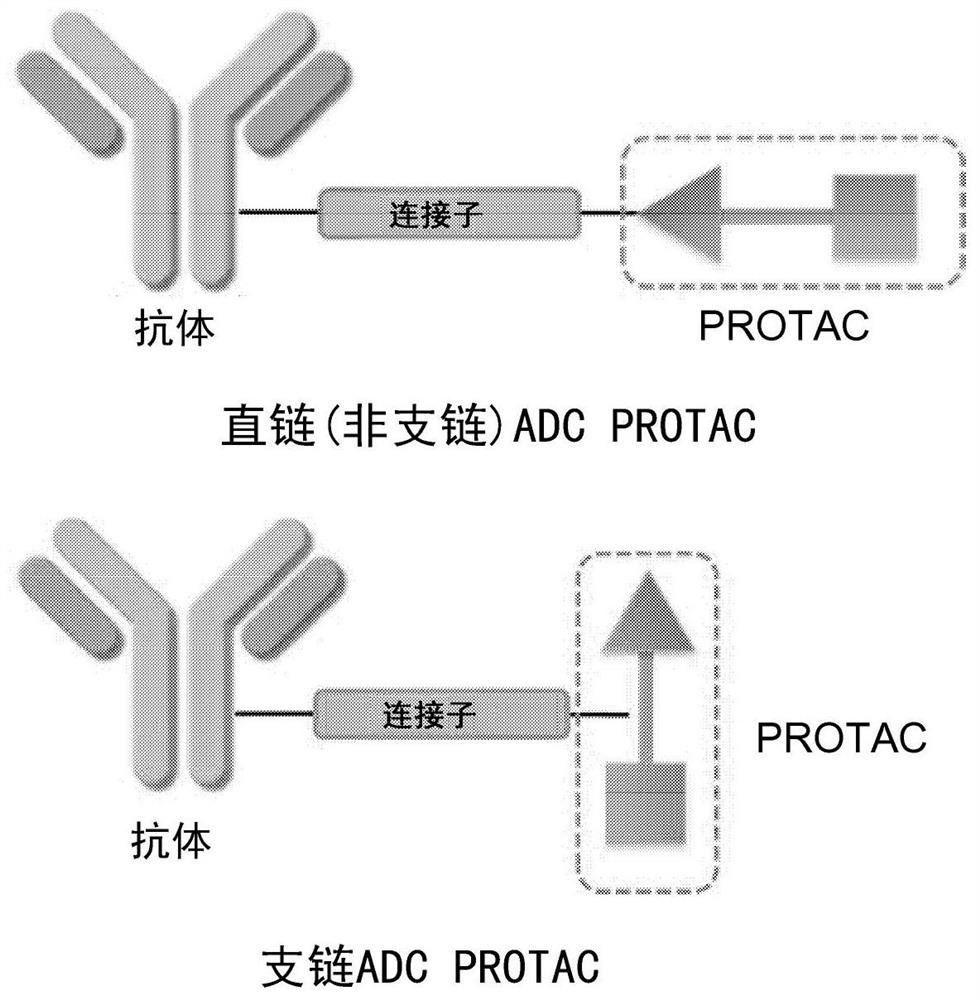
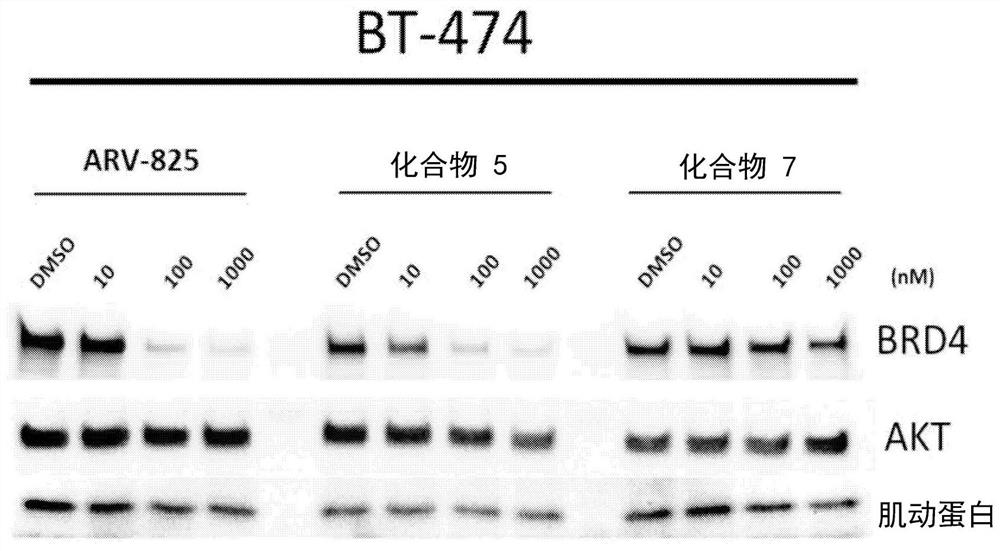
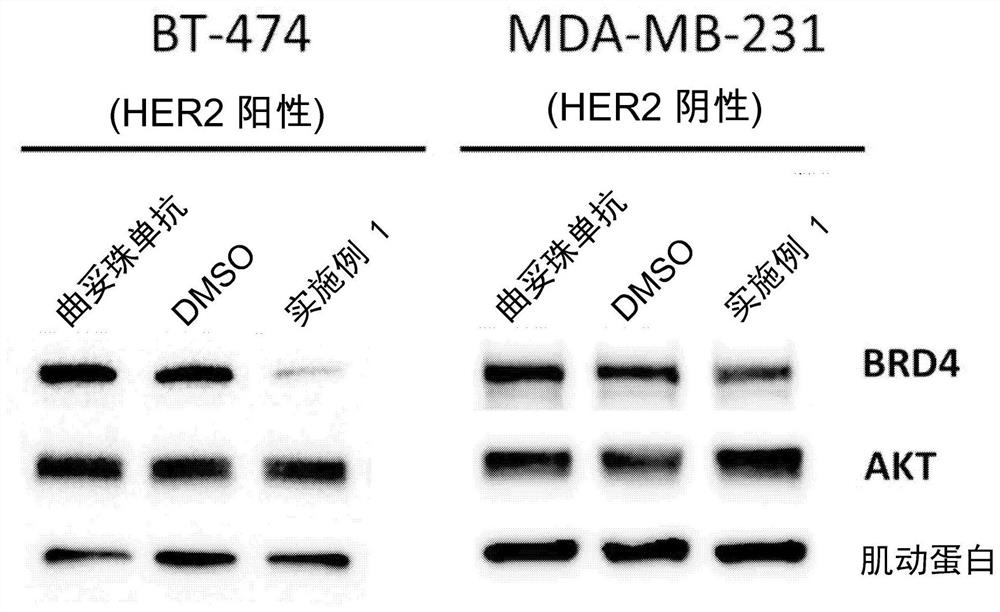
Antibody protac conjugates
A technology of immunoconjugates and antibodies, applied in the direction of antibodies, anti-animal/human immunoglobulins, anti-tumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Preparation of Trastuzumab-BRD4-PROTAC-1
[0051] Synthesis of compound 2
[0052]
[0053] In this example, the BET inhibitor is OTX015, an orally bioavailable small molecule inhibitor of BRD2, BRD3, and BRD4 (EC 50 = 10 to 19 nM). OTX015 downregulates c-Myc expression and induces cell cycle arrest and apoptosis. Therefore, it has anti-proliferative effects on various solid tumors and leukemia.
[0054] Compound 2: To a mixture of OTX015(1) (0.2 mmol) and 1-bromo-2-(2-bromoethoxy)ethane (1 mmol) in dimethylformamide (5 mL) was added potassium carbonate (0.6 mmol). The mixture was stirred at 50°C for 24 hours. After the reaction was completed, the reaction mixture was extracted with dichloromethane and water. Then, the organic layer was washed with brine and washed with MgSO 4 dry. The organic solvent was removed under reduced pressure. The residue was purified by column chromatography with methanol:dichloromethane (1:19) to afford compound 2 as a...
Embodiment 2
[0067] Example 2: Preparation of Trastuzumab-BRD4-PROTAC-2
[0068] Synthesis of Compound 8
[0069]
[0070] Compound 8: To a solution of 4-(N-maleimidomethyl)cyclohexane-1-carboxylic acid succinimide (SMCC) (0.75 mmol) in acetonitrile (7 mL) was added 1, 2-ethanedithiol (0.82 mmol). The reaction mixture was stirred at room temperature for 3 hours. After the reaction was completed, the reaction mixture was extracted with dichloromethane and water. Then, the organic layer was washed with brine and washed with MgSO 4 dry. The organic solvent was removed under reduced pressure. The residue was purified by column chromatography with ethyl acetate:hexane (3:2) to afford compound 8 as a white solid (34.8% yield).
[0071] Synthesis of compound 9
[0072]
[0073] Compound 9: To a solution of compound 7 (0.02 mmol) in acetonitrile / dimethylformamide (1:1, 6 mL) was added compound 8 (0.04 mmol). The reaction mixture was stirred at room temperature for 16 hours. After th...
Embodiment 3
[0078] Example 3: Synthesis of a linear form of BRD4-PROTAC (17)
[0079] Figure 4 A possible synthetic scheme via the A-conjugated synthesis of ARV-825 is illustrated. Functional group modification of protein ligands or ligase conjugates is not easy. Furthermore, not all protein ligands or ligase conjugates have suitable functional groups for modification. In this example, the chlorine atom on OTX015 (the protein ligand of PROTAC ARV-825) was difficult to convert into another functional group, such as amino group. OTX015 or ARV-825 showed no reactivity under Buchwald reaction (palladium catalyzed coupling reaction) and Ullman reaction (copper catalyzed coupling reaction). Harsh reaction conditions, such as metal halide exchange, will lead to compound decomposition. According to the literature (EP1887008A1), different BRD4 inhibitor functional groups should be introduced at the outset. In other words, direct coupling of linkers to protein ligands or ligase conjugates wou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com