Photocurable composition for PCB containing alkenyl ether and/or oxetane compounds and free radical polymeric compounds
A polymeric compound and light-curable technology, applied in applications, household utensils, inks, etc., can solve the problems of short light source life, poor performance of light-cured film, ozone pollution and other problems, and achieve steep imaging side walls and good curing performance Excellent, improve the effect of surface polymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0138] According to one embodiment of the present invention, the photocurable composition comprises at least one other cationically polymerizable compound different from component (a2).
[0139] The other cationic polymer compounds may be in the form of monomers or resins such as oligomers or prepolymers. According to one embodiment of the present invention, the other cationic polymerizable compound may be selected from oxirane compounds and aziridine compounds.
[0140] The oxirane compound may be selected from, for example, glycidyl ether epoxy compounds, glycidyl ester epoxy compounds, glycidyl amine epoxy compounds, aliphatic epoxy compounds, alicyclic epoxy compounds and the like. The oxirane compounds may be in the form of monomers or resins such as oligomers or prepolymers. There may also be mentioned compounds having both an oxirane group and a radically polymerizable group such as an acrylate group, such as epoxy (meth)acrylate resins. Preferable are glycidyl ether ...
Embodiment 1-77 and comparative example 1-3
[0241] Infrared light-curable inkjet printing ink According to the above preparation method, the inkjet printing inks of Examples 1-77 and Comparative Examples 1-3 were respectively prepared according to the components and proportions in Table 1-Table 8 below.
[0242] Table 1 Formula table of infrared light-curable inkjet printing ink
[0243]
[0244] Table 2 Formula table of infrared light-curable inkjet printing ink
[0245]
[0246]
[0247] Table 3 Formula table of infrared light-curable inkjet printing ink
[0248]
[0249]
[0250] Table 4 Formula table of infrared light curing inkjet printing ink
[0251]
[0252]
[0253] Table 5 Formula table of infrared light-curable inkjet printing ink
[0254]
[0255]
[0256] Table 6 Formula table of infrared light-curable inkjet printing ink
[0257]
[0258]
[0259] Table 7 Formula table of infrared light curing inkjet printing ink
[0260]
[0261]
[0262] Formulation table of the...
Embodiment 78
[0368] Example 78: Reaction of vinyl ether monomers and methacrylate monomers in the presence of free radical photoinitiators
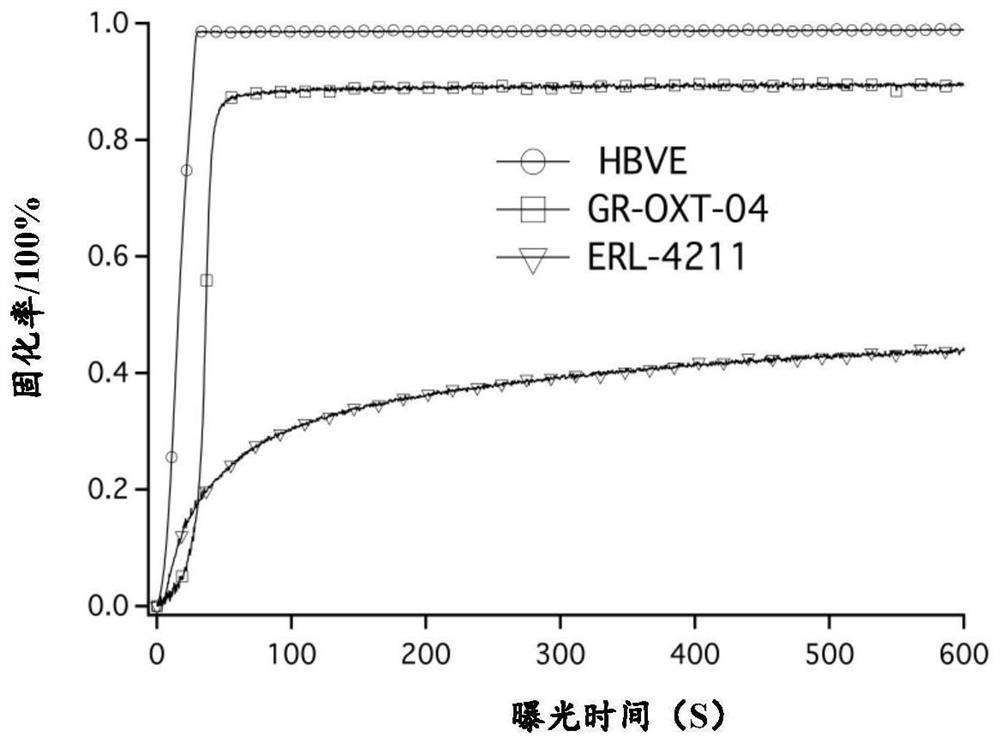
[0369] Using high-efficiency free radical photoinitiator bis(2,4,6-trimethylbenzoyl)phenylphosphine oxide (trade name 819) as free radical photoinitiator, using 395nm UV-LED light source (light intensity 100mW / cm 2 ), using the Photo-DSC test method to test the initiation effect of 819 on acrylate free radical polymerizable monomer TPGDA and 4-hydroxybutyl vinyl ether monomer (HBVE). The experimental results are attached figure 1 (Plot of sample heat flow versus exposure time). The experimental results show that when the light intensity is 100mW / cm 2 When 819 was used to initiate TPGDA polymerization under the 395nm UV-LED light source, the heat flow curve of the sample changed significantly, which was caused by the heat released by the cross-linking reaction of the double bond of TPGDA. There was hardly any change in the heat flow of the sample, i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com