Process for recovering non-ferrous metals from industrial mineral residues
A non-ferrous metal and residue technology, applied in the direction of improving process efficiency, can solve the problems of high Fe dissolution rate, difficulty in Zn recovery, etc., and achieve the effect of environmental friendliness and cost avoidance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Mixture Ratio
[0040] The starting materials goethite and jarosite sludge with the chemical composition listed in Table 1 were mixed together in different weight ratios (1:1, 1:2, 1:3 and 1:4), and Sulfated roasting.
[0041] Table 1: Chemical composition of starting goethite and jarosite sludge (dried at 40 °C for 24 h)
[0042]
[0043] Calcination was performed by heating the mixture to 700°C at a rate of 120°C / h at atmospheric pressure in an open reactor (ie, a reactor open to the outside atmosphere). After 1 hour at 700°C, the samples were cooled and ground manually in a mortar and pestle to break up aggregates formed during firing. The chemical composition of the mixture obtained after sulfation roasting is listed in Table 2.
[0044] Table 2 Chemical composition of tested goethite-jarosite mixtures after calcination at 700°C
[0045]
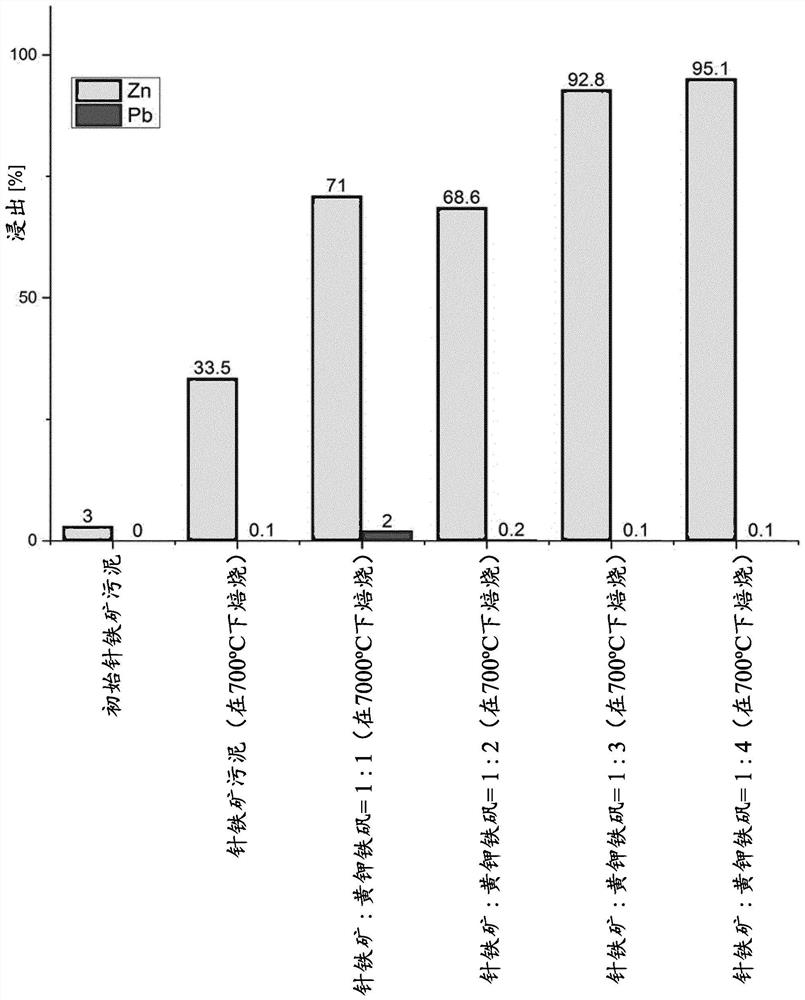
[0046] These mixtures after sulphation roasting were leached in water for selective leaching of Zn. Water ...
Embodiment 2
[0053] Example 2: Post-processing steps
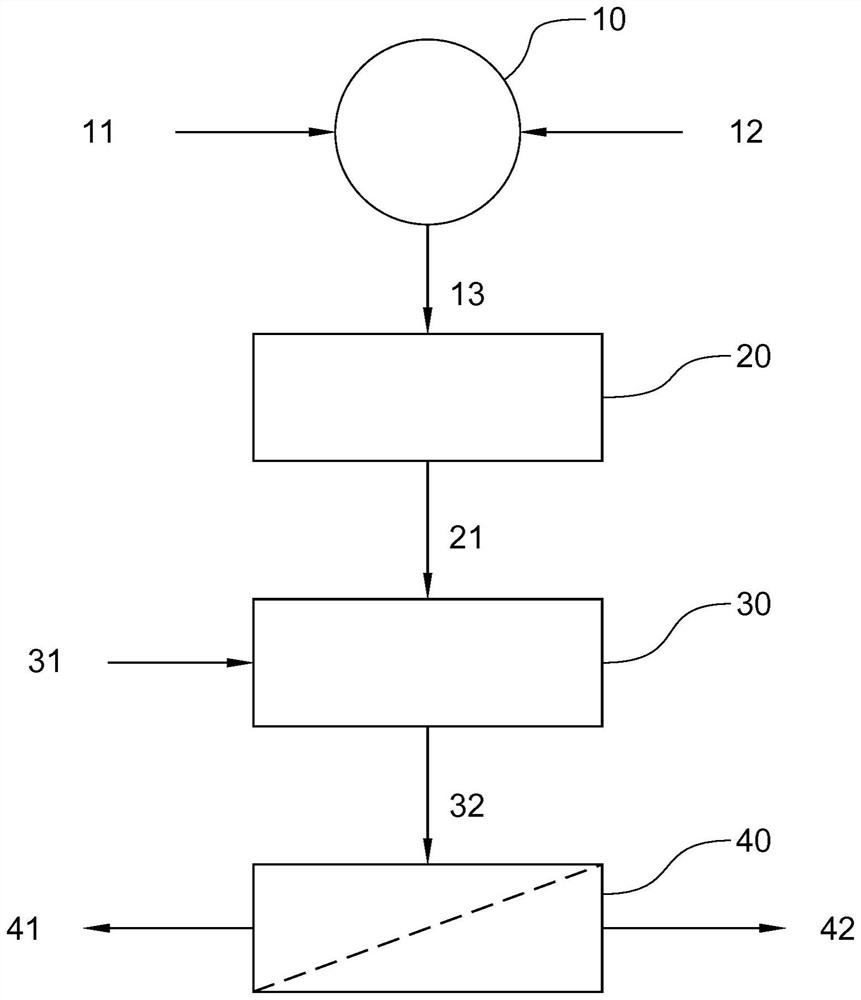
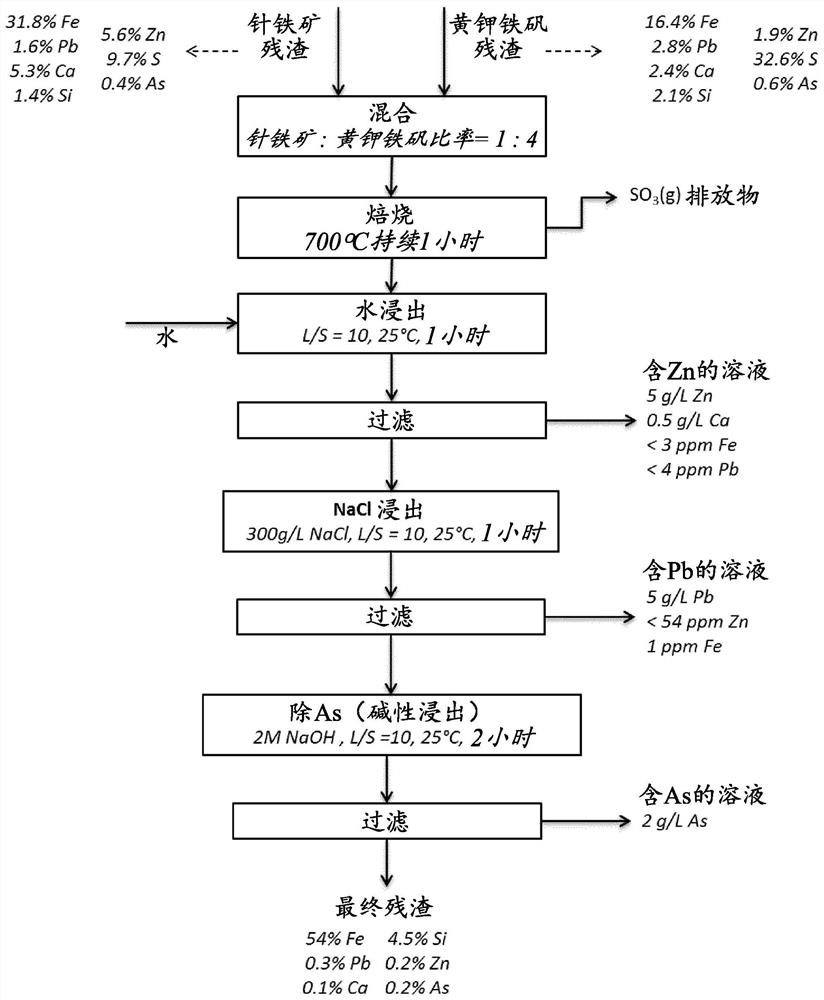
[0054] The solid residue as obtained from Example 1 can be further processed by a selective leaching step, eg to remove Pb and As. A suitable flowchart is in image 3 shown in , especially for a mixture of goethite and jarosite in a ratio of 1:4. The mixing, roasting and water leaching steps are the same as in Example 1 above.
[0055] A first filtration step 40 is performed after water leaching to separate the leachate 41 , which is a zinc-rich solution, from the zinc-depleted solid material 42 . The leachate contained 5 g / L Zn, 0.5 g / L Ca and less than 3 ppm Fe and less than 4 ppm Pb.
[0056] A subsequent leaching step 50 is performed on the solid material 42 . The solid material 42 was leached in a salt solution of 300 g / L NaCl at L / S=10 at 25° C. for two hours. The leach suspension 51 from the leaching step 50 is fed to a filtration step 60 to separate a Pb-rich leachate 61 and a Pb-depleted solid residue 62 . The leachate 6...
Embodiment 3
[0058] Embodiment 3: the influence of roasting temperature on pollutant leaching
[0059] Using jarosite as the sulphur-containing residue, it has been found that this material will bind toxic elements like arsenic and prevent their leaching into leach solutions containing ferrous metals like zinc. For example, arsenic is typically known as arsenate (AsO 4 3-) in waste streams. During roasting, it will partially replace the jarosite structure Pb 0.5 Fe 3 (SO 4 ) 2 (OH) 6 SO in 4 2- . as from Figure 4 It is clear from the diagram that roasting can influence the (further) incorporation of such pollutants in the residue structure in a positive manner. Firing temperature can be an important parameter. refer to Figure 4 , at roasting temperatures starting from about 675°C, there was no detectable leaching of arsenic into the leachate from water leaching after roasting of pure jarosite residues (all process conditions identical to Example 2).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com