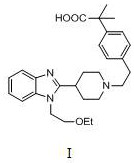
Preparation method of bilastine important intermediate
A technology of benzimidazole and piperidine, which is applied in the field of medicinal chemistry, can solve the problems of long synthesis reaction time and achieve the effects of less side reactions, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
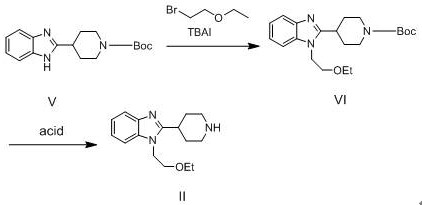
[0017] Add 2.0 g of Compound V and 20 mL of THF to the bottle, and start stirring. Slowly add 0.8g NaH, react at room temperature for 1 hour. Add 1.0 g of tetrabutylammonium iodide and 4.1 g of bromoethyl ethyl ether, heat to reflux, and react for 1 hour. 20 mL of water was added to quench the reaction, and THF was removed by rotary evaporation. The residue was extracted with DCM 3 times, using 20 mL each time, separated, and the organic phase was collected. The organic phase was spun evaporated to remove DCM. PE 8 mL was added to the residue and stirring was started. Ice bath for 1 hour. After suction filtration, the resulting solid was air-dried at 50°C for 6-8 hours to obtain 2.3 g of compound VI as a white solid, with a yield of 92.8%.
[0018] After adding 2.8 mL of concentrated hydrochloric acid to the system, add 28 mL of deionized water and stir. Add 2.0 g of compound VI into the system and continue to stir, and react for more than 3 hours. The reaction was moni...
Embodiment 2
[0020] Add 6.0 g of Compound V and 60 mL of THF to the bottle, and start stirring. Slowly add 2.4g NaH, and react at room temperature for 1 hour. Add 3.0 g of tetrabutylammonium iodide and 4.1 g of bromoethyl ethyl ether, heat to reflux, and react for 1 hour. The reaction was quenched by adding 60 mL of water, and THF was removed by rotary evaporation. The residue was extracted with DCM for 3 times, using 60 mL each time, the layers were separated, and the organic phase was collected. The organic phase was spun evaporated to remove DCM. PE 25 mL was added to the residue and stirring was started. Ice bath for 1 hour. After suction filtration, the resulting solid was air-dried at 50°C for 6-8 hours to obtain 6.5 g of compound VI as a white solid, with a yield of 87.4%.
[0021] After adding 8.4 mL of concentrated hydrochloric acid to the system, add 84 mL of deionized water and stir. Add 6.0 g of compound VI into the system and continue to stir, and react for more than 3 h...
Embodiment 3
[0023] Add 20.0 g of Compound V and 200 mL of THF to the bottle, and start stirring. Slowly add 8.0 g of NaH and react at room temperature for 1 hour. Add 10.0 g of tetrabutylammonium iodide and 41.0 g of bromoethyl ethyl ether, heat to reflux, and react for 1 hour. 200 mL of water was added to quench the reaction, and THF was removed by rotary evaporation. The residue was extracted with DCM for 3 times, using 200 mL each time, the liquid was separated, and the organic phase was collected. The organic phase was spun evaporated to remove DCM. PE 80 mL was added to the residue and stirring was started. Ice bath for 1 hour. After suction filtration, the resulting solid was air-dried at 50°C for 6-8 hours to obtain 23.4 g of compound VI as a white solid, with a yield of 94.4%.
[0024] After adding 28.0 mL of concentrated hydrochloric acid to the system, add 280 mL of deionized water and stir. Add 20.0 g of compound VI into the system and continue to stir, and react for more...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com