Application of compound shown in formula I in preparation of drug for treating obesity and related symptoms
A technology for related diseases and compounds is applied in the field of use of the compound of formula I in the preparation of medicines for the treatment of obesity and related diseases, which can solve the problems of easy rebound, easy problems with insulin, and increased diabetes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
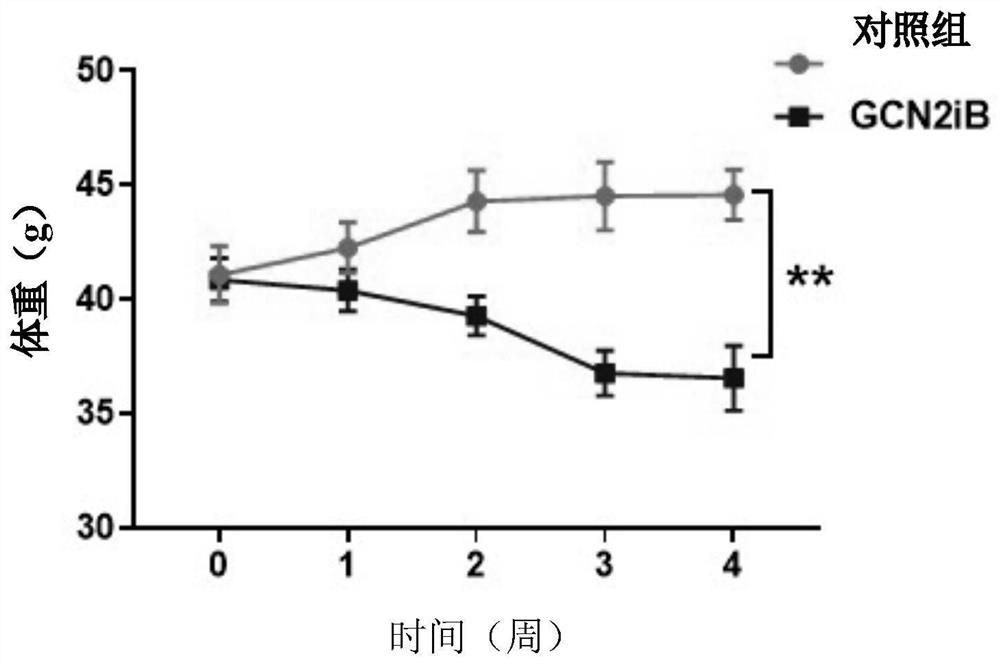
[0045] Taking 8-10 week-old wild-type C57BL6 mice as research objects, fed with high-fat diet with a fat content of 62% by weight for 3 months, they were randomly divided into blank control group and GCN2iB treatment group, 5 mice in each group, two groups There was no significant difference in the initial weight of the figure 1 shown. On the first day of modeling, the drug GCN2iB was dissolved in olive oil and administered to mice in the GCN2iB treatment group by intragastric administration at a dose of 5 mg / (kg body weight) every 2 days for 4 weeks. The blank control group was given the same volume of olive oil every 2 days for 4 weeks. During the administration period, the mice in the two groups continued to be fed with high-fat diet, and their body weight was weighed every week. After the experiment, blood was taken from the eyeballs of the mice, and biochemical and histological analyzes were performed on the liver at the same time. The experimental results were as follo...
Embodiment 2
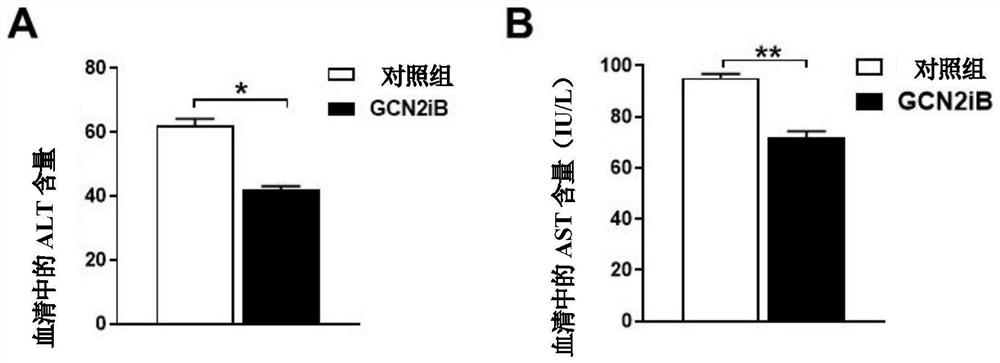
[0047] The homozygous mouse for leptin gene mutation (Lepob, usually written as ob or ob / ob) is a classic animal model of non-alcoholic fatty liver disease. Ob / ob mice aged 10-12 weeks were randomly divided into blank control group and GCN2iB treatment group, 5 mice in each group. There was no significant difference in body weight among the groups. The administration method and dosage are the same as in Example 1, and the duration is 6 weeks. Body weight was recorded during the experiment. At the end of the experiment, blood was taken from the eyeballs of the mice, and biochemical and histological analyzes were performed on the liver. The experimental results are as Figure 4 , Figure 5 A-5B and Image 6 A-6C shown. from Figure 4 It can be seen that oral administration of GCN2iB significantly reduced the body weight of ob / ob mice (* represents p Figure 5 In A and 5B, it can be seen that oral administration of GCN2iB significantly reduced alanine aminotransferase (ALT) ...
Embodiment 3
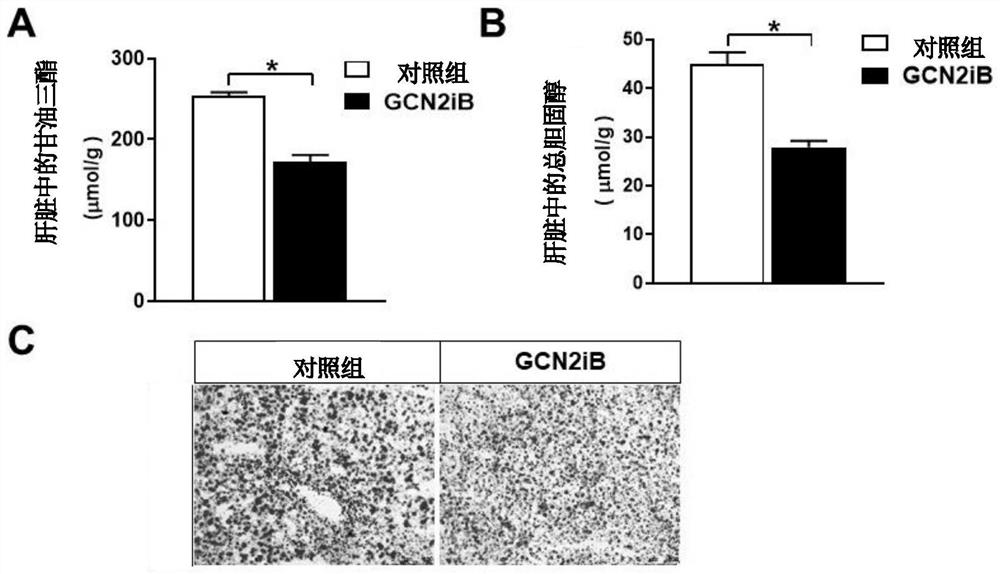
[0049] Leptin receptor mutant db / db mice have typical clinical symptoms of diabetes such as extreme obesity, polyphagia, thirst, and polyuria, and are also a typical animal model of fatty liver. db / db mice aged 6-8 weeks were selected and randomly divided into blank control group and GCN2iB treatment group with similar body weight. The administration method and dosage are the same as in Example 1, and the duration is 4 weeks. After the experiment, blood was taken from the eyeballs of the mice, and biochemical analysis was performed on the liver at the same time. The experimental results are as Figure 7 , Figure 8 A-8B and Figure 9 A-9C shown. from Figure 7 It can be seen that oral administration of GCN2iB can maintain the body weight of db / db mice basically unchanged, while the weight of db / db mice in the control group increased significantly (** represents p Figure 8 In A and 8B, it can be seen that oral administration of GCN2iB can significantly reduce the alanine a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com