Transaminase mutant and applications of transaminase mutant in Sitagliptin synthesis
A mutant and transaminase technology, applied in the fields of genetic engineering and enzyme catalysis, can solve the problems of expensive reagents, chiral catalysts and chiral auxiliary agents, etc., and achieve the effect of improving enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] The screening of embodiment 1 wild-type transaminase
[0069] 1. Enzyme mining and whole gene synthesis
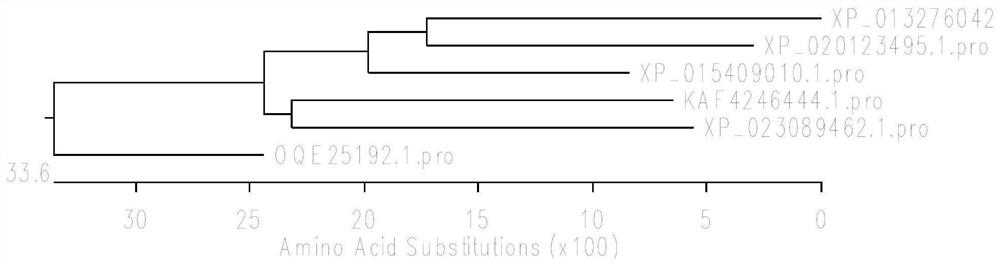
[0070] Using bioinformatics analysis technology, six strains of (R)-ω-transaminase with potential catalytic ability of aromatic ring and its derivatives were excavated from NCBI database: OQE25192.1 (Penicillium steckii), XP_015409010.1 (Aspergillus nomiae), KAF4246444.1 (Aspergillus fumigatiaffinis), XP_013276042.1 (Rhinocladiella mackenziei), XP_023089462.1 (Aspergillus oryzae), XP_020123495.1 (Talaromyces atroroseus), sequence differences are as follows figure 1 shown.
[0071] The nucleic acid sequence was optimized according to the codon preference of Escherichia coli, and after the gene was synthesized, it was cloned into the NcoI and XhoI sites of the plasmid pET22b, and a His tag was added to the C-terminus of the amino acid sequence. Plasmids pET-PSATA(OQE25192.1), pET-ANATA(XP_015409010.1), pET-AFATA(KAF4246444.1), pET-PMATA(XP_013276042.1), pET-AOATA(XP...
Embodiment 2
[0086] Embodiment 2 Transaminase AFATA key site mutation and screening
[0087] 1. Construction of mutants
[0088] Using bioinformatics technology to analyze AFATA, it is determined that T57, R77, K179, E212, I237, T238 and T273 in the amino acid sequence are located in the substrate-cofactor binding region, G50, F51, H53, G54, L56, T57, K84, E115, I117, W147, L181, D185, T187, F191, D205 are dimer polypeptide binding interfaces, and these sites play key roles in the structure and function of the enzyme.
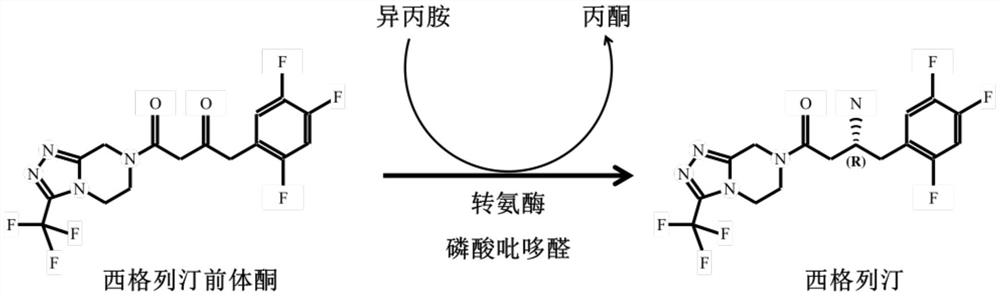
[0089] Since the sitagliptin precursor ketone contains both 1,2,4-triazolo[4,3-a]pyrazine and 2,4,5-trifluorophenyl structures, it reduces transaminase and substrate binding sites resistance. R77, K179, and E212 are related to enzyme catalysis and cofactor binding, and G50 and G54 are relatively small in steric hindrance and will not be changed. The remaining 16 sites were divided into three groups for combined mutations. The first group of mutations: F51V, T57AFG, I117A...
Embodiment 3
[0163] Example 3 The second round of mutation and screening of AFATA key sites
[0164] 1. The second round of mutant construction
[0165] Referring to the method of Example 2, based on the amino acid sequence shown in SEQ ID NO.3, the histidine at position 53 is mutated to leucine (H53L); or the threonine at position 57 is mutated to glycine (T57G) ; or mutation of 84th lysine to histidine (K84H); or mutation of 117th isoleucine to alanine (I117A); or mutation of 191st phenylalanine to alanine (F191A) ; or the 205th aspartic acid mutation to serine (D205S); or the 273th threonine mutation to serine (T273S).
[0166] For the H53L mutation, design forward and reverse primers:
[0167] Forward primer H53L-51: 5'-TGGATGAAGGCTTTATGCTTGGCGATGCGACC-3'
[0168] Reverse primer H53L-31: 5'-GGTCGCATCGCCAAGCATAAAGCCTTCATCCA-3'
[0169] For the T57G mutation, design forward and reverse primers:
[0170] Forward primer T57G-51: 5'-ATGCATGGCGATGCGGGCTATGATGTGACCAC-3'
[0171] Reverse...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com