Bacteriostatic protein and encoding gene and application thereof and strain method
A technology of encoding genes and proteins, applied in the field of antibacterial proteins and their encoding genes, uses and strains, can solve the problems of poor antibacterial effect and limited application, and achieve the effect of improving antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, lysozyme mutant design
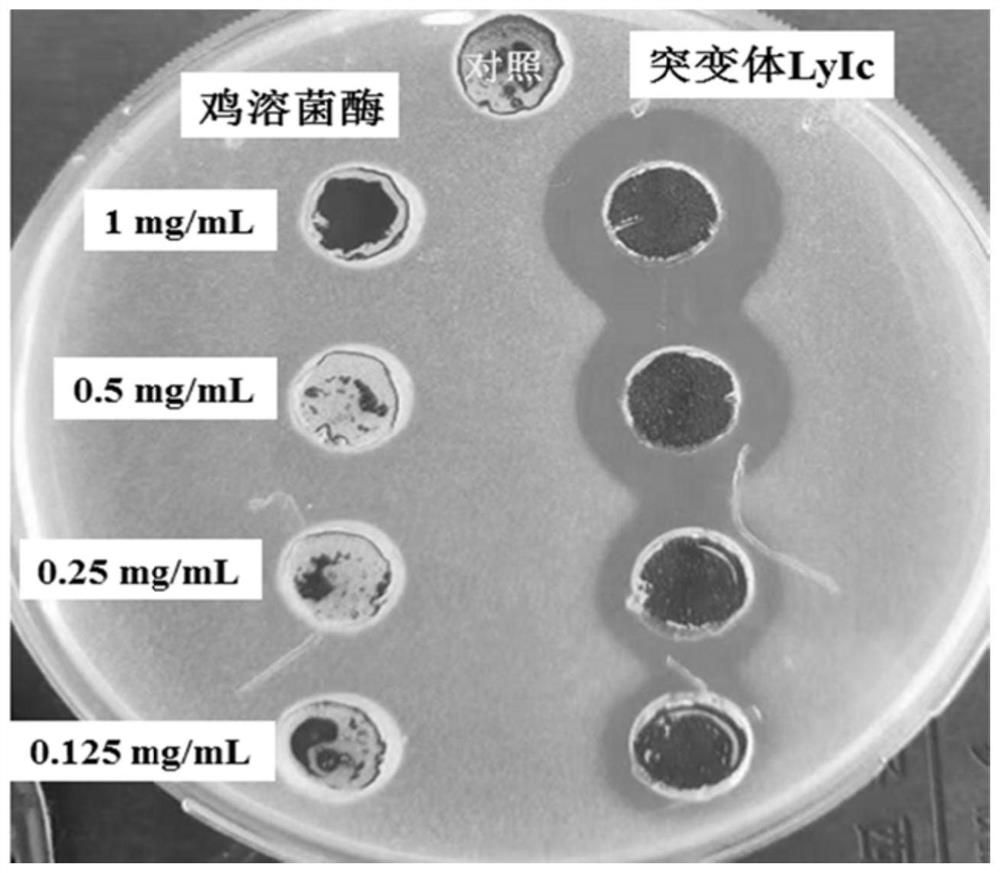
[0034] Studies have shown that superimposing antimicrobial peptides on the basis of lysozyme amino acids can improve the antibacterial effect of lysozyme. In our previous research, we obtained a scorpion-derived antimicrobial peptide IsCT (CN104263749A) with good antibacterial effect through experiments. This patent hopes to pass The amino acid sequence of the antimicrobial peptide IsCT was superimposed on chicken lysozyme protein to improve the antibacterial properties of chicken lysozyme. The design process of the chicken lysozyme mutant LyIc containing the antimicrobial peptide IsCT is as follows: (1) In order to prevent the amino acid sequence of the antimicrobial peptide IsCT from wrapping inside the mutant LyIc protein and affecting its antibacterial activity, the amino acid sequence of the antimicrobial peptide IsCT was The sequence is superimposed on the N-terminus of the chicken lysozyme protein; (2) Due to the strong anti...
Embodiment 2
[0035] Embodiment 2, chicken lysozyme mutant LyIc gene synthesis and expression vector construction
[0036] According to the codon preference of Pichia pastoris, the nucleotide sequence encoding the mutant LyIc was optimized by online codon optimization software to obtain the gene lys-Is, the nucleotide sequence of which is shown in SEQ ID NO.1. The gene lys-Is is 486bp in full length (without signal peptide), encoding 161 amino acids, including antioxidant peptide (DEDTQAMP), antimicrobial peptide IsCT (ILGKILKGIKKLF), amino acid linker (GGGSSSSGGGG) and chicken lysozyme (129 amino acids). The optimized gene lys-Is was obtained by General Biosystems (Anhui) Co., Ltd. through the whole gene synthesis method. A pair of primers were designed according to the sequence of lys-Is (primer sequences were fw: 5'-agtc gaattcGATGAAGACACTCAAGCTATG-3' and rev: 5'-ttctctaga TTACAATCTACAAC CTCTGAT-3') for the amplification of gene lys-Is, agarose electrophoresis Detect the PCR amplificat...
Embodiment 3
[0037] Embodiment 3, construction and screening of recombinant Pichia pastoris
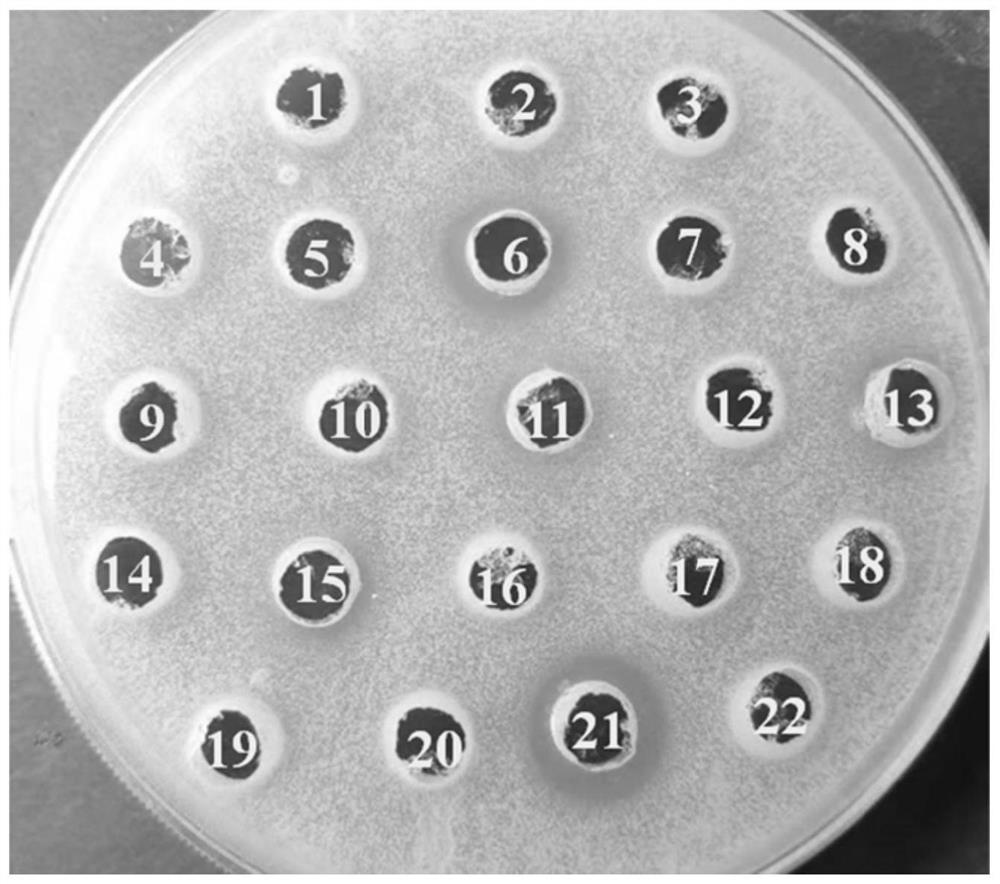
[0038]The expression vector pPICZαA-lys-Is was linearized with SacI and then transformed into Pichia pastoris X33, and the recombinant transformants were spread on Zeocin resistance plates with different concentrations (100 μg / ml-500 μg / ml). Use a toothpick to pick the yeast recombinant transformants grown on the plate one by one into a 24-well plate containing 2mL of BMGY medium in each well, culture at 30°C, 220rpm for about 36h, and then add 1% (v / v) methanol for induction culture . After culturing at 30°C and 220rpm for 48 hours, the supernatant was collected by centrifugation for antibacterial assay. The specific steps of the antibacterial test are as follows: (1) Cultivate Micrococcus luteus (CICC10680) to the logarithmic growth phase (OD600 is about 1), and add the cultured bacterial solution to the sterile medium at 1 μL / mL to prepare flat. (2) Use a hole puncher with a radius of 3 mm t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com