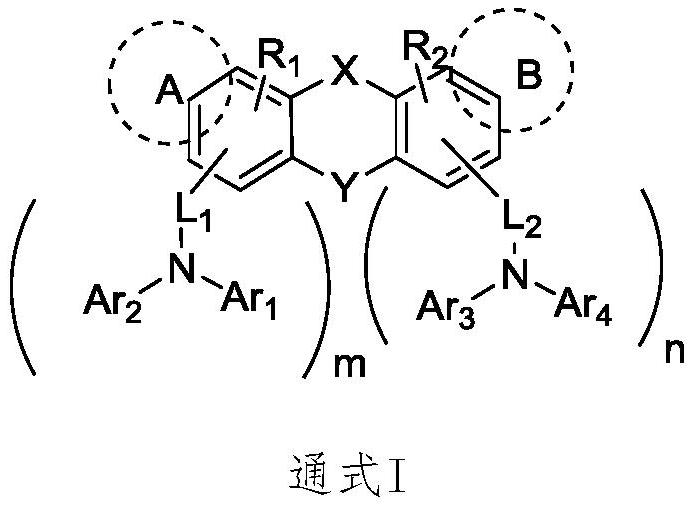
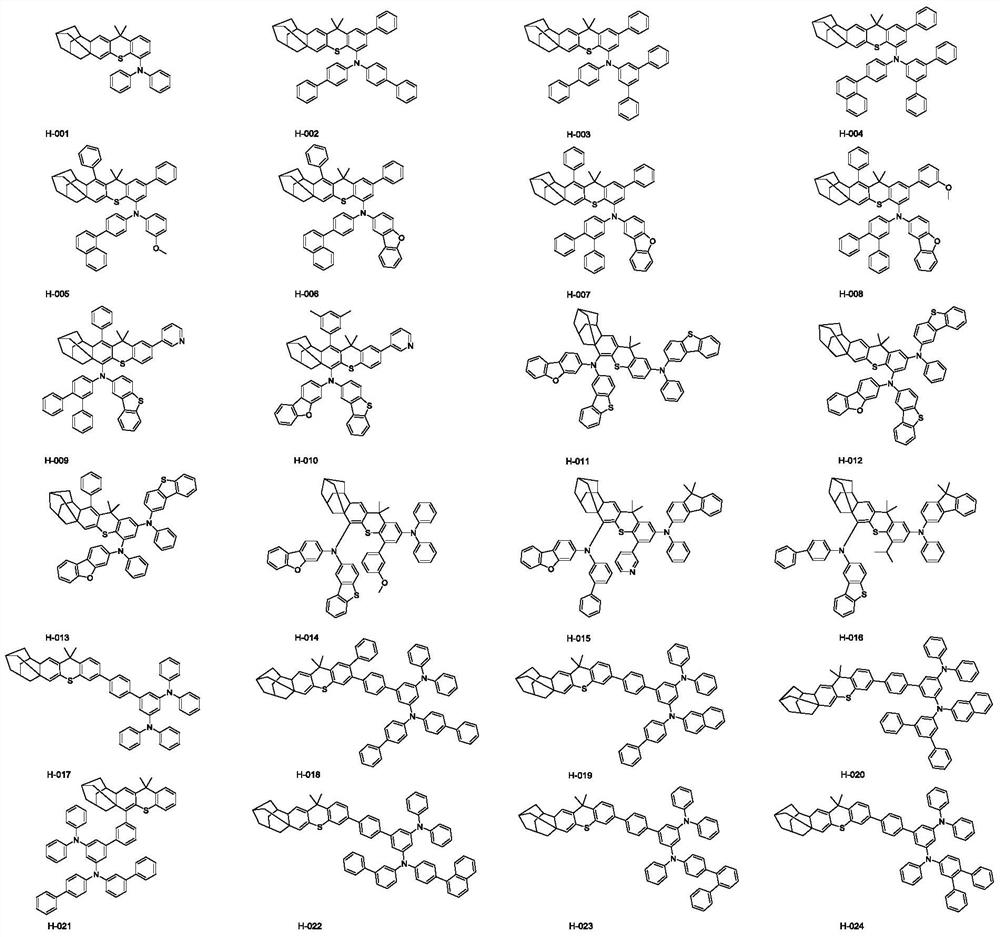
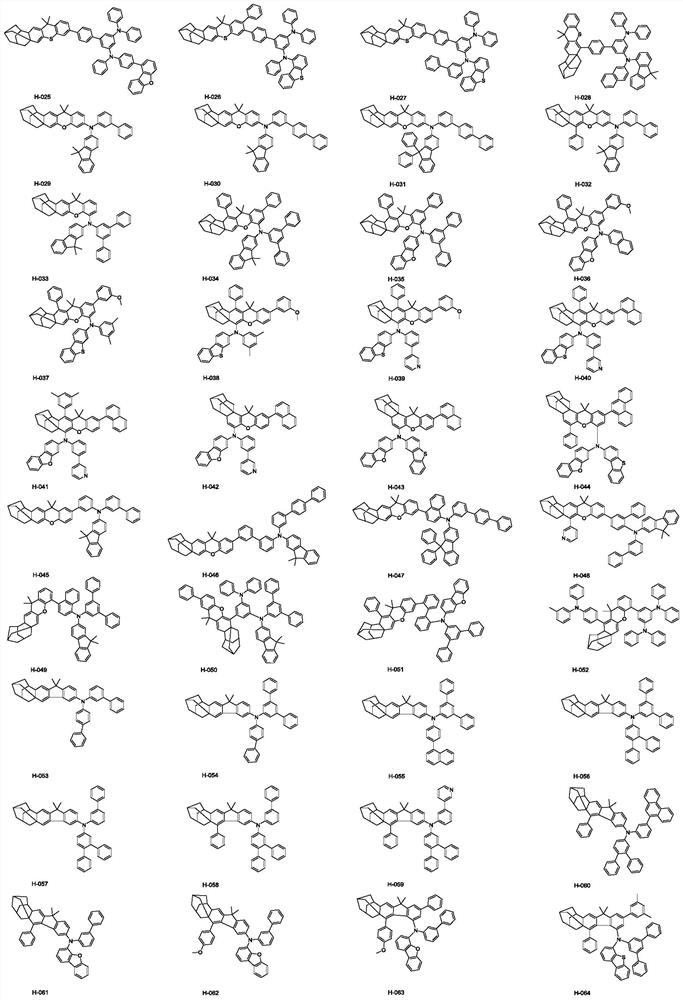
Hole organic electroluminescent compound as well as preparation method and application thereof
A compound and luminescence technology, which is applied in the preparation of organic compounds, amino compounds, organic chemistry, etc., can solve the problems of low driving voltage and unsatisfactory life, and achieve life extension, maximum external quantum efficiency improvement, drive The effect of voltage reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: preparation compound H-006
[0059] synthetic route:
[0060]
[0061] Specific synthesis method:
[0062] Step 1. Under nitrogen protection, dissolve raw material A-006 (11.31g, 20.00mmol) and raw material E-006 (7.71g, 20.00mmol) in 180.00ml of toluene solution, add tris(dibenzylideneacetone) di Palladium (0.18g, 0.20mmol), tri-tert-butylphosphine (0.20g, 1.00mmol) and sodium tert-butoxide (3.84g, 40.00mmol), stir evenly, heat up to 90°C, and reflux for 5h; after the reaction , slightly lower the temperature to 70°C, filter with diatomaceous earth to remove salt and catalyst, wash the filtrate three times with water after cooling to room temperature, keep the organic phase, then extract the aqueous phase with ethyl acetate; after combining the organic phase, use Magnesium sulfate water was dried, and the solvent was removed using a rotary evaporator; using dichloromethane and petroleum ether (V 二氯甲烷 :V 石油醚 =10:4) was purified by column chromatogra...
Embodiment 2
[0069] Embodiment 2: preparation compound H-048
[0070] synthetic route:
[0071]
[0072] Specific synthesis method:
[0073] Step 1, under nitrogen protection, raw material A (9.49g, 20mmol) and raw material B (5.54g, 20mmol) were dissolved in 150.00ml toluene, ethanol and water (V 甲苯 :V 乙醇 :V 水 =3:1:1) into the mixed solution, add tetrakis triphenylphosphine palladium (0.23g, 0.20mmol) and potassium carbonate (5.53g, 40.00mmol), stir well, heat up to 90°C, and reflux for 5 hours, After the solution was cooled to room temperature, the organic phase was retained, and then the aqueous phase was extracted with ethyl acetate; after the organic phases were combined, they were dried using anhydrous magnesium sulfate, and the solvent was removed using a rotary evaporator to obtain solid organic matter. Use about 100ml of dichloromethane to completely dissolve the solid organic matter, then slowly add it dropwise into the petroleum ether solution, stir evenly, and a precipit...
Embodiment 3
[0081] Embodiment 3: preparation compound H-052
[0082] synthetic route:
[0083]
[0084]
[0085] Specific synthesis method:
[0086] Step 1, under nitrogen protection, raw material A-052 (14.29g, 30.00mmol) and raw material B-052 (6.02g, 30.00mmol) were dissolved in 200.00ml toluene, ethanol and water (V 甲苯 :V 乙醇 :V 水 =3:1:1) in the mixed solution, add tetrakis triphenylphosphine palladium (0.35g, 0.30mmol) and potassium carbonate (8.29g, 60.00mmol), stir well, heat up to 90°C, and reflux for 5 hours, After the solution was cooled to room temperature, the organic phase was retained, and then the aqueous phase was extracted with ethyl acetate; after the organic phases were combined, they were dried using anhydrous magnesium sulfate, and the solvent was removed using a rotary evaporator to obtain solid organic matter. Use 100ml of dichloromethane to completely dissolve the solid organic matter, then slowly add it dropwise into the petroleum ether solution, stir eve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com