A kind of enzyme-sensitive nano system targeting T cells and its preparation method and application
An enzyme-sensitive and systematic technology, applied in the field of medicine, to achieve high drug loading and good drug loading capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Synthesis of Stearoyl Modified Polypeptides
[0058] The stearyl-modified polypeptide: stearyl-HHHRRRRPPLGLAGK-Mal, was synthesized by Zhejiang Hongtuo Co., Ltd. using the peptide solid-phase synthesis method and named sHRP. The synthesized sHRP was purified by preparative high-performance liquid chromatography, and its purity reached more than 95%. Wherein HRP is a polypeptide, and the amino acids are connected by peptide bonds to form 15 peptides ( figure 1 , figure 2 ).
Embodiment 2
[0059] Example 2: Method for co-loading chemical drugs and antibodies in sHRP micelles
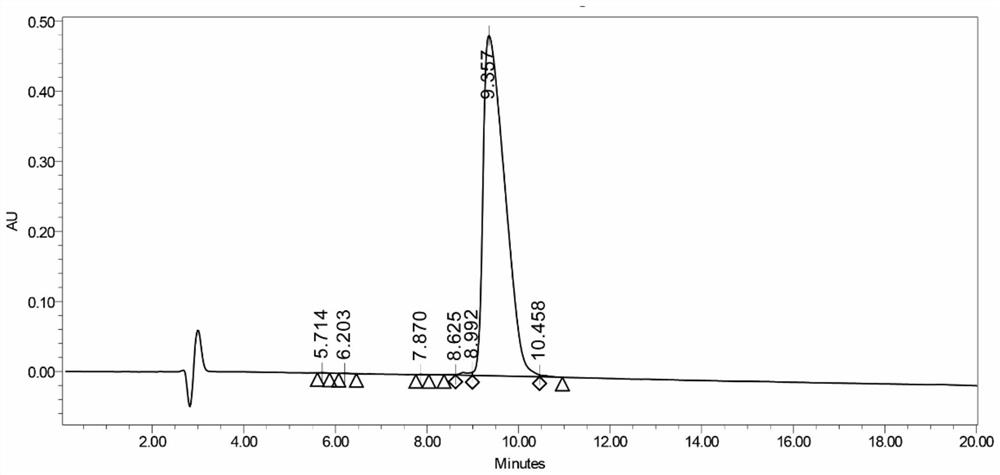
[0060] Dissolve sHPR in water and CH223191 in dichloromethane. The volume ratio of water and dichloromethane is 1:5. After mixing and stirring for 2 hours, open the cover and keep stirring to volatilize the dichloromethane completely. Prepare CH-sHRP micelles. The obtained micellar solution was mixed with the thiolated CD28 antibody solution, the mass ratio of micelles to antibody was 1:10, and the reaction was sealed in the dark for 24 hours to obtain co-sHRP. Average particle size results and potential see image 3 , 4 . The particle size results are consistent with the TEM images.
Embodiment 3
[0061] Example 3: Investigation of the in vitro release characteristics of CH223191
[0062] Dialysis bag method was used to further evaluate the release degree of CH-sHRP with and without MMP. A dialysis bag with a relative molecular mass of 1000 was selected, and the dialysis medium was PBS solution with pH=7.4. Put MMP-treated CH-sHRP and untreated CH-SHRP into dialysis bags, put them in 50mL of dialysate, 100r / min, at 2, 4, 6, 8, 10, 12, 24, 36, 48h At the time point, 1 mL of external fluid was taken, and 1 mL of dialysate was added, the concentration was measured by HPLC, and the in vitro release curve was drawn. Such as Figure 6 It is shown that in the presence of MMP, the release of CH223191 is faster, which is because the fragmentation of the MMP-sensitive peptide in the structure of the carrier leads to a decrease in the particle size of the nanostructure, thereby accelerating the release.
PUM
| Property | Measurement | Unit |
|---|---|---|
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com