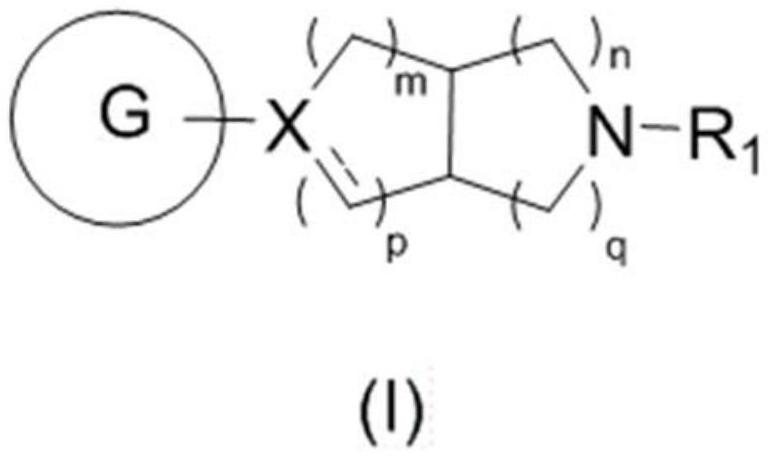
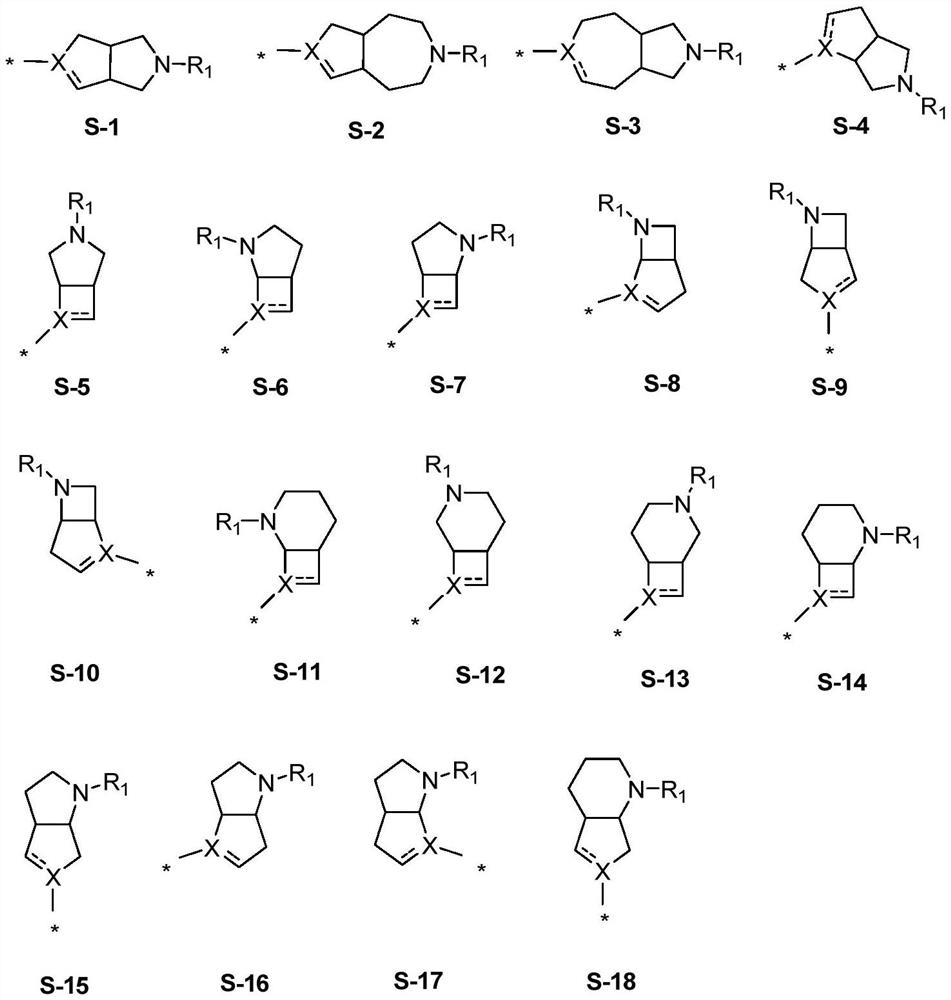
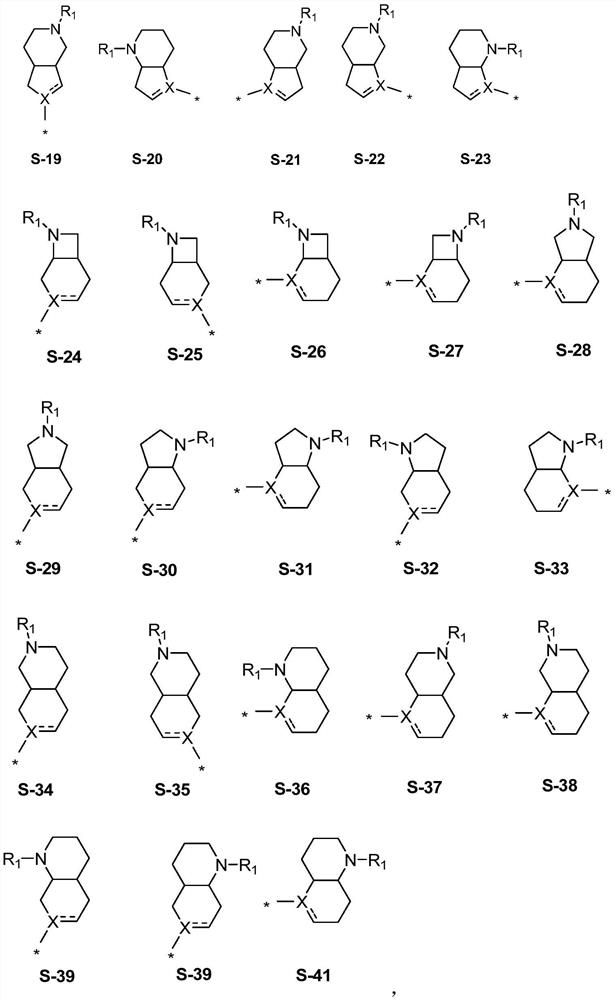
Fused ring compound and preparation method and application thereof
A compound and alkyl technology, applied in the field of medicinal chemistry, can solve the problems of no curative effect and low curative effect for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] Example 1: 2-naphthalene-2-yl octahydropyrrole [3,4-c] pyrrole
[0119]
[0120] step 1:
[0121] 2-Bromonaphthalene 1-a (10.7g, 52mmol, 1.1eq), hexahydropyrrolo[3,4-c]pyrrole-2(1H)-carboxylic acid tert-butyl ester (10g, 47mmol, 1eq) was added to 150mL of toluene , adding Pd to the toluene solution 2 (dba) 3 (2.16g, 2.36mmol, 0.05eq), BINAP (2.35g, 3.78mmol, 0.08eq), potassium tert-butoxide (7.9g, 70.8mmol, 1.5eq), reaction N 2 Protection, heating at an external temperature of 105 degrees for 10 hours. Dilute the reaction solution with 150ml ethyl acetate, filter, wash the filter residue with a small amount of ethyl acetate, wash the filtrate with 300ml saturated sodium chloride solution, dry the organic phase with anhydrous sodium sulfate, and spin dry to obtain 16g of brown oil, add 100ml of ethanol to the oil to make a slurry , a large amount of solids were precipitated, stirred at room temperature for 2 hours, the solids were uniformly dispersed, filtered, an...
Embodiment 2
[0124] Example 2: 2-methyl-5-(naphthalene-2-yl)octahydropyrrole[3,4-c]pyrrole
[0125]
[0126] step 1:
[0127] The product of Example 1 (7.5g, 31.6mmol) was added to 120ml of ethyl formate, triethylamine (17.6ml, 126.6mmol, 4eq) was added, and the system was heated at an external temperature of 60°C for 3h. 100ml of ethyl acetate was diluted, and the organic phase was washed with 200ml of saturated ammonium chloride solution, then washed with 200ml of saturated sodium chloride solution, dried over anhydrous sodium sulfate, and spin-dried to obtain compound 2-a, 8.4g of light yellow solid, yield 99.8%.
[0128] Step 2:
[0129] Compound 2-a (8.4g, 31.6mmol) was added to 120ml of dry tetrahydrofuran, and 1M borane tetrahydrofuran solution (63.2ml, 63.2mmol, 2eq) was added, and the reaction solution gradually dissolved, N 2 Under protection, the external temperature is 68 degrees for 2 hours. The reaction solution was ice-bathed, and 7.9ml of hydrochloric acid (3eq) was ...
Embodiment 3
[0130] Example 3: 5-(Benzo[b]thiophen-4-yl)octahydrocyclopentane[c]pyrrole hydrochloride
[0131]
[0132] step 1:
[0133] Add thionyl chloride (1.2g, 10mmol) dropwise to a solution of 3-a (2.27g, 10mmol) and triethylamine (1.01g, 10mmol) in dichloromethane (30mL) at 0°C, and rise to React at 20°C for 5 hours. Water was added to quench the reaction, the layers were separated, the organic layer was concentrated and subjected to column chromatography (EA:PE=1:15 to 1:5) to obtain compound 3-b (1.25 g, yield 60%).
[0134] Step 2:
[0135] At 0°C, add potassium tert-butoxide to a THF (30 mL) solution of bis-boronic acid pinacol ester (2.54 g, 10 mmol), cuprous chloride (25 mg, 0.25 mmol) and ligand xantphos (145 mg, 0.25 mmol) ( 1.12g, 10mmol), under nitrogen protection, raised to 20°C and reacted for 30min after the addition, then added dropwise a THF (2mL) solution of 3-b (1.23g, 5mmol); reacted overnight at room temperature. The reaction solution was directly added to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com