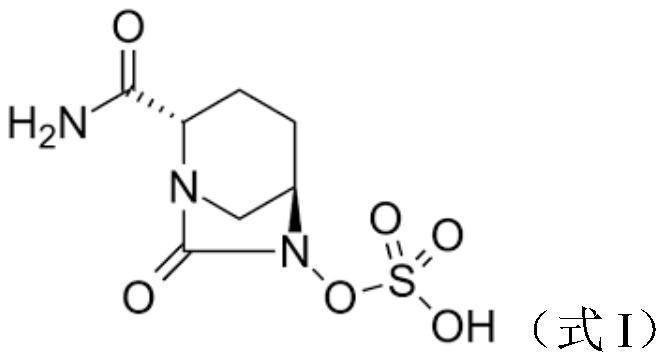
Antibacterial composition containing avibactam and meropenem and application thereof
A meropenem and multipurpose technology, applied in the field of medicine, can solve the problems of reduced clinical effectiveness, non-inhibitable metallo-beta-lactamases, and decreased bacterial sensitivity to drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
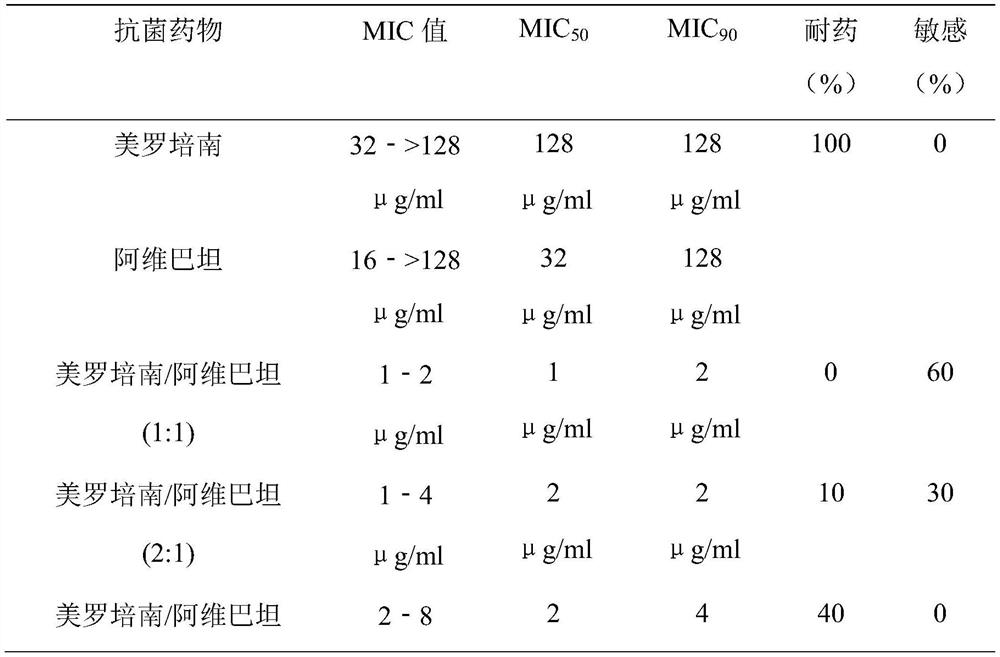
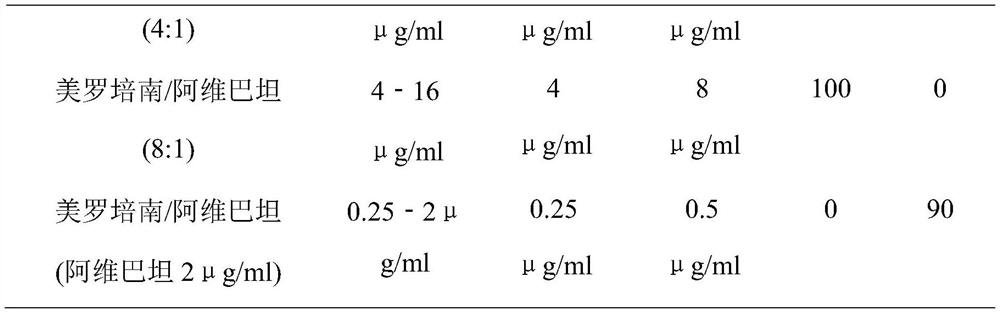
[0049] Antibacterial activity of meropenem-avibactam
[0050] 1. Antibacterial drugs
[0051] Meropenem (trihydrate) and avibactam sodium were mixed in different concentration ratios (1:1, 2:1, 4:1, 8:1) and fixed concentrations of avibactam (2 μg / ml, 4 μg / ml , 8 μg / ml) mixed to determine the joint antibacterial activity.
[0052] 2. Test bacteria
[0053] Clinically isolated strains producing β-lactamase genotypes of KPC, NDM, OXA, IMP, and VIM.
[0054] 3. Experimental protocol
[0055] According to CLSI (Clinical and Laboratory Standards Institute) M07-A10 (2015 edition), the minimum inhibitory concentration of antibacterial drugs such as meropenem and avibactam against clinical isolates was determined by the Broth-micro dilution method. (MIC). The combined antibacterial activity of different concentrations and ratios was determined by 12×12 checkerboard dilution method (Checkerboard) combined with drug susceptibility test.
[0056] 3.1 Preparation of antibacterial dr...
Embodiment 2
[0077] Antibacterial activity of meropenem-avibactam
[0078] According to the method in Example 1, three groups of meropenem / avibactam concentration ratios of 1:1 and 2:1 and a fixed concentration of avibactam (4 μg / ml) were selected, and the total number of clinical strains below 1050 were determined. Antimicrobial Activity: MRSA, Staphylococcus aureus, Staphylococcus epidermidis, MRSA, MRSA, MRSA, MRSA, MRSA Staphylococcus, Enterococcus faecium, Vancomycin-resistant Enterococcus faecium, Enterococcus faecalis, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Enterobacter cloacae, carbapenems sensitive or resistant Acinetobacter baumannii, Burkholderia cepacia, Alcaligenes, Stenotrophomonas maltophilia), Haemophilus influenzae (Haemophilus parafluidus, Haemophilus influenzae β- Lactamase (-), Haemophilus influenzae β-lactamase (+)) Citrobacter freundii, Proteus mirabilis, Proteus vulgaris, Morganella morganii, Providencia sp., Serrat ...
Embodiment 3
[0098] Drug efficacy experiment in mice
[0099] 1. Antibacterial drugs
[0100] Get the corresponding medicines and prepare the following 6 groups of medicinal liquids with normal saline:
[0101] Table 5 drug groups
[0102]
[0103] 2. Test bacteria
[0104] Clinical strain of Klebsiella pneumoniae (provided by Huashan Hospital Affiliated to Fudan University);
[0105] 3. Experimental protocol
[0106] 3.1 Determination of Bacterial Toxicity
[0107] Each bacterium was diluted into serial concentrations, and 0.5 ml of ICR mice (body weight 20±2 g) were infected intraperitoneally, observed for 7 days, and the survival of the animals was observed. Results: Klebsiella pneumoniae clinical strain-26 was the most toxic (KPC-producing type).
[0108] 3.2 Determine the minimum lethal concentration (MLD) of the strain:
[0109] Klebsiella pneumoniae clinical strain 26 was diluted into serial concentrations, and 0.5 ml of ICR mice (body weight 20±2 g) were infected intraper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com