Megestrol acetate nanocrystalline oral mixed suspension as well as preparation method and application thereof
A technology of megestrol acetate and oral suspension, which is applied in the field of medicine and can solve the problems of great post-suffering pain and long-term stability to be improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
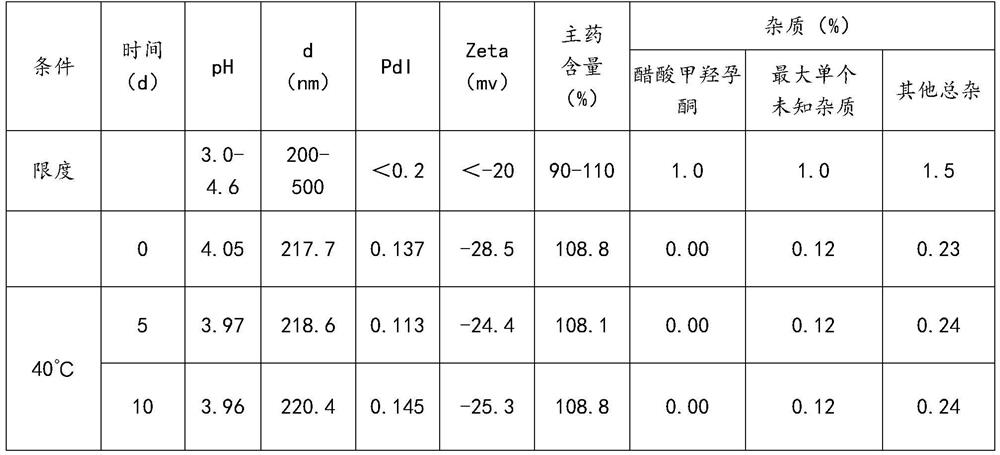
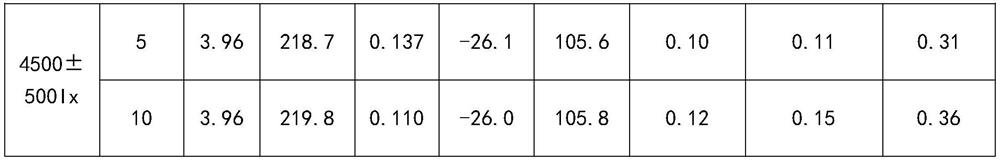
[0074] Example 1 Preparation of Megestrol Acetate Nanocrystalline Oral Suspension
[0075] The composition of megestrol acetate nanocrystalline oral suspension is shown in Table 1.
[0076] Table 1
[0077] components Dosage (g) Megestrol Acetate (MA) 120.2 HPMC (E5) 24 Docusate Sodium (DOSS) 1.2 sodium benzoate 1.9 Sodium citrate dihydrate 1.8 Citric Acid Monohydrate 4 sucrose 144.2 Lemon Spice GLR101378 1.5 Lemon Spice GLR101340 0.5 water 700.7
[0078] The preparation method of embodiment 1 comprises the following steps:
[0079] Weigh 24g of HPMC E5 and add appropriate amount of water, heat to 75°C, stir until clear and transparent, let stand at room temperature, and set aside. Weigh 1.2g of DOSS, add an appropriate amount of water, stir until dissolved, add 1.9g of sodium benzoate, 1.8g of sodium citrate dihydrate and 4g of citric acid monohydrate, stir until dissolved, and prepare a mixed solu...
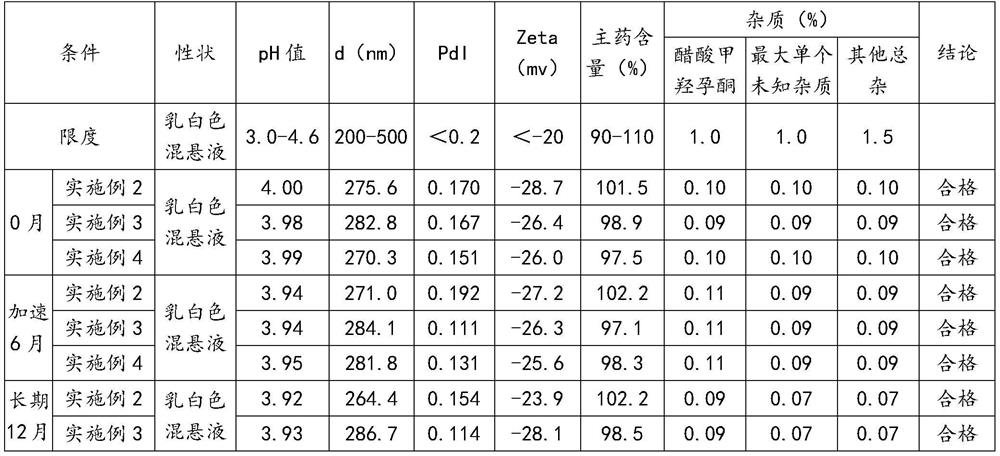
Embodiment 2-4
[0087] The preparation of embodiment 2-4 megestrol acetate nanocrystal oral suspension
[0088] The composition of embodiment 2-4 megestrol acetate nanocrystal oral suspension is shown in Table 3.
[0089] table 3
[0090] components Dosage (g) megestrol acetate 346.8 HPMC E5 69.2 Docusate Sodium (DOSS) 3.46 sodium benzoate 5.48 Sodium citrate dihydrate 5.19 Citric Acid Monohydrate 11.54 sucrose 416.0 Lemon Spice GLR101378 4.33 Lemon Spice GLR101340 1.44 water 2021.5
[0091] The preparation method of embodiment 2-4 comprises the steps:
[0092] Weigh 69.28g HPMC E5 and add appropriate amount of water, heat to 80°C, stir until clear and transparent, let stand at room temperature, and set aside. Weigh 3.46g DOSS, add appropriate amount of water, stir until dissolved, add 5.48g sodium benzoate, 5.19g sodium citrate dihydrate and 11.54g citric acid monohydrate, stir until dissolved, and prepare a mixed so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com