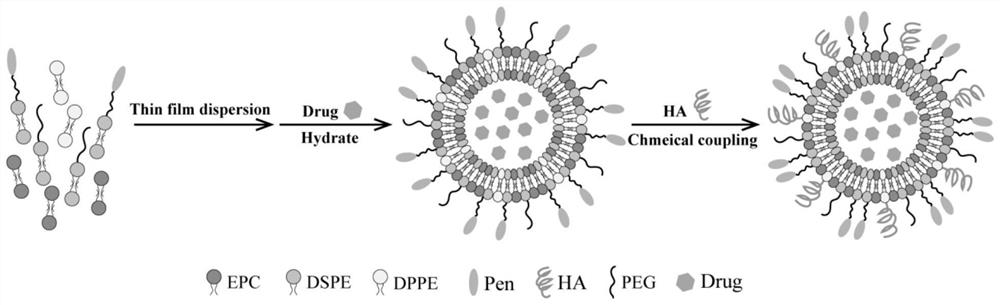
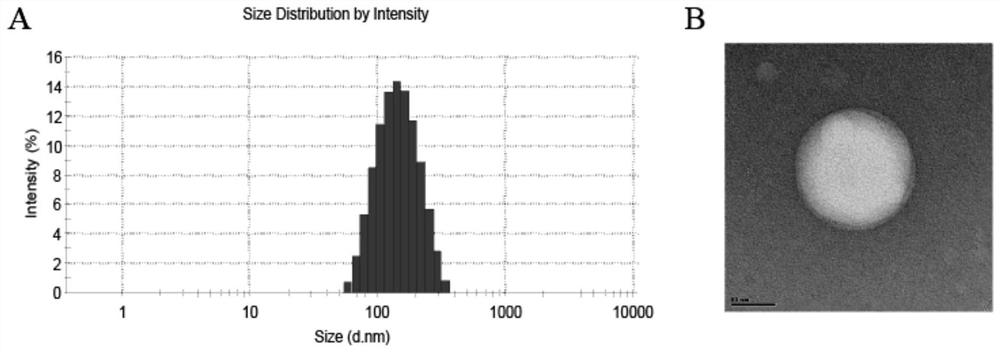
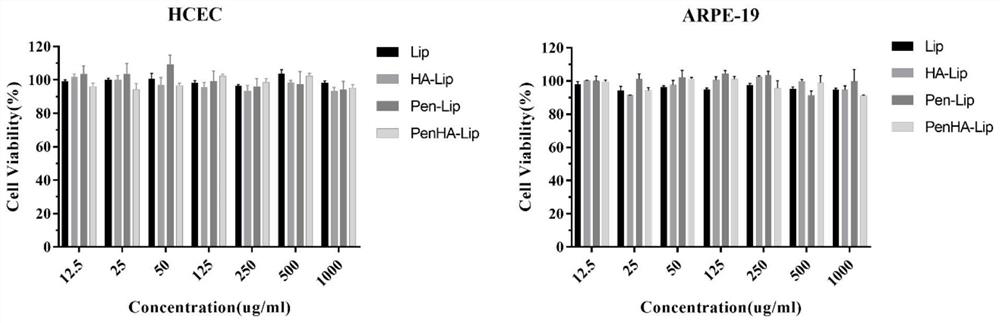
Ophthalmic liposome capable of penetrating cornea and targeting retina as well as preparation method and application of ophthalmic liposome
An ophthalmic liposome and liposome technology, applied in the field of medicine, can solve the problems of high eye irritation, poor fluidity of unimproved carrier, inability to effectively improve vitreous fluidity and retinal targeting, and achieve The effect of improving medication adherence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1. Preparation of liposomes
[0048] (1) Link DSPE-PEG-Mal with cell penetrating peptide CPP by addition reaction.
[0049] The cell penetrating peptide CPP was synthesized by Shanghai Qiangyao Biotechnology Co., Ltd., and CPP was thiolated. CPP is specifically
[0050] Penetratin: RQIKIWFQNRRMKWKK (SEQ ID NO: 1), wherein C is an amino acid-cysteine C added during the sequence synthesis, and this amino acid contains a sulfhydryl group, that is, the sulfhydrylation of CPP is completed.
[0051] DSPE-PEG-CPP is obtained by 1,4 addition reaction between the maleimide group of DSPE-PEG-Mal and the sulfhydryl group on the cysteine residue of the cell penetrating peptide CPP. DSPE-PEG-Mal (11.6 mg) and thiolated Penetratin (16.8 mg) were dissolved in chloroform solution, triethylamine was added dropwise as a catalyst, and shaken gently. The mixed solution was reacted at room temperature and protected from light for 24 h under the condition of nitrogen filling. The org...
Embodiment 2
[0076] During the research process, our research group investigated the influence of multiple factors on the therapeutic effect of liposomes, including the following CPP and HA ratio screening experiments.
[0077] Human corneal epithelial cells HCEC were divided into 5 × 10 per well 6 The density of cells was seeded into the upper chamber of the collagen-coated Transwell chamber to construct a simulated in vitro corneal barrier. Retinal pigment epithelial cells ARPE-19 per well 2 × 10 5 The density of cells was plated in the lower chamber of the Transwell to simulate the fundus retinal environment. In order to investigate the efficiency of liposomes modified with different ratios of CPP and HA (the preparation method is the same as in Example 1) to penetrate the corneal barrier and be taken up by retinal cells, the fluorescently labeled drug-loaded liposomes modified with different ratios of CPP and HA were added to Go to the upper chamber of the Transwell chamber. After b...
Embodiment 3
[0083] Comparison of intraocular fluorescence intensity of different vectors.
[0084] PenHA-Lip: Penetratin, HA double modified liposome set; prepared according to the method of Example 1.
[0085] PenHA-PAMAM: Penetratin, HA double-modified PAMAM group; the preparation method is: first thiolate Penetratin, then take the thiolated Penetratin (16.8mg) and NHS-PEG-Mal (8.7mg) and dissolve them in 3mL of phosphate buffer and vortex After spinning for 1 min, it was added dropwise to 6 mL of PAMAM (10 mg) in phosphate buffer, and Pen-PAMAM was obtained after reaction. HA (22mg), EDC (22mg), and NHS (22mg) were dissolved in acetate buffer, and preactivated at 37°C for 2h. The activated HA was then added to Pen-PAMAM (35.5mg), adjusted to a final pH of 8 with borate buffer, and incubated at 37°C for 12h, stirring with a magnetic stirrer to couple HA to the liposome surface , forming PenHA-PAMAM.
[0086] PenHA-PLGA: Penetratin, HA double-modified PLGA group; the preparation metho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com